Fig 10. Implications of the high HBc phosphorylation—Low CLP RNA content correlation for specific pgRNA encapsidation in HBV infection.
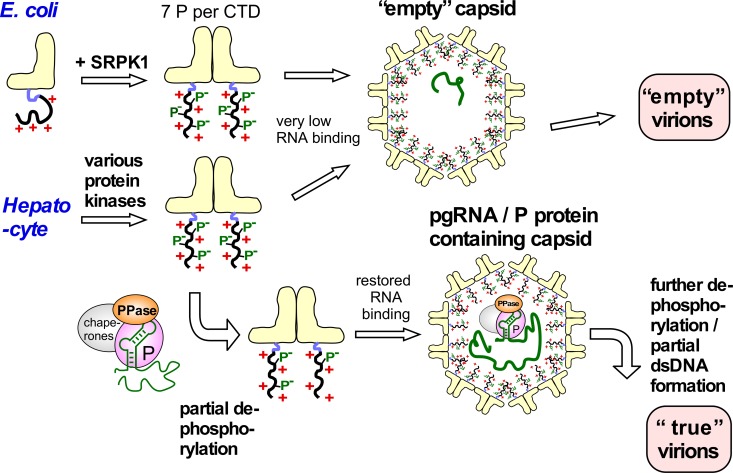
In E.coli nonphosphorylated HBc183 CTDs have maximal positive charge and thus maximal electrostatic RNA binding capacity, generating RNA-filled CLPs (Fig 1B). Seven-fold phosphorylation by SRPK1 neutralizes most positive charges, leading to virtually empty capsids. The similarly strong Phos-tag retardation of most HBc183 from human cells indicates similarly high phosphorylation, whether by SRPK1 or a combination of kinases. Strongly reduced RNA binding favors formation of empty capsids and then empty virions, perhaps the dominant pathway in vivo [52]. The backside of avoiding irrelevant RNA packaging is a loss in specific pgRNA interaction capability. As blocking just one of the seven SRPK1 phosphorylation sites restored substantial RNA packaging in bacteria we propose that the pgRNA/P protein complex, besides other host factors, also carries a protein phosphatase (PPase) activity. This PPase would dephosphorylate only nearby HBc CTDs, and thus locally unleash their RNA binding potential in proximity to pgRNA. This would go on with further HBc dimers until the shell is completed. Progressive dephosphorylation of the now internal CTDs by the PPase activity could maintain electrostatic homeostasis, especially during the near doubling of negative charges associated with dsDNA formation. Targeted nucleocapsid destabilization upon infection of a new cell could occur by CTD re-phosphorylation and completion of plus-strand DNA.