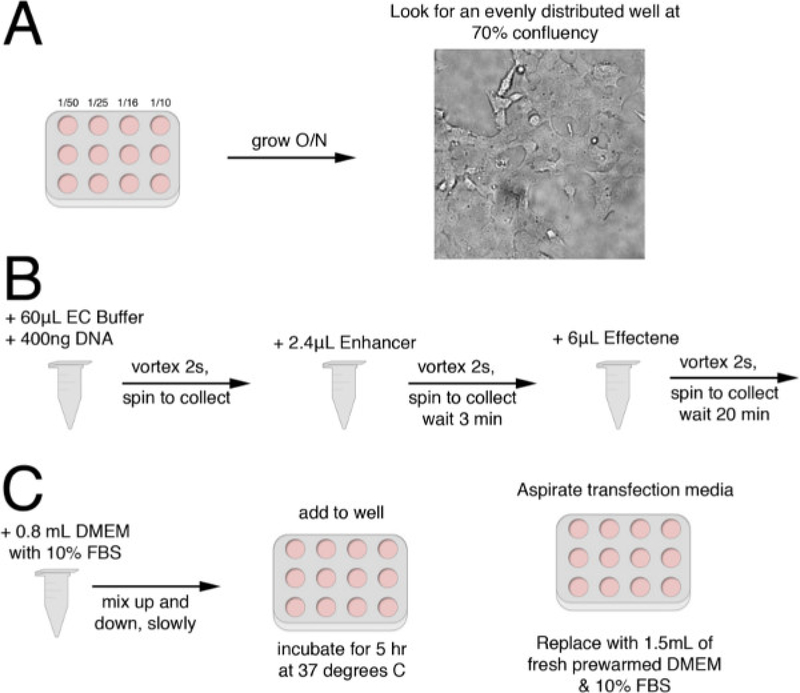
Figure 1: Flow Chart of Transfection Procedure.
(A) First, seed a variety of dilutions (1:50, 1:25, 1:16, 1:10) onto a 12-well plate. Allow cells to grow overnight. The next day, look for a well that is about 70% confluent, and evenly distributed, as depicted. This is the well that will be transfected. (B) Add 60 μl of EC buffer and 400 ng of DNA into a microcentrifuge tube. Vortex for 10 sec and spin the liquid to bottom of the tube. Add 2.4 μl of Enhancer. Vortex for 2 sec and spin the liquid to bottom of the tube. Incubate at room temperature for 3 min. Add 6 μl of Effectene. Vortex for 2 sec and spin the liquid to bottom of the tube. Incubate at room temperature for 20 min. (C) Slowly mix 0.8 ml of DMEM with 10% FBS with the transfection. Add to cells in previously selected well. Incubate cells for 5 hr at 37 °C. Replace with fresh DMEM with 10% FBS.