Figure 2.
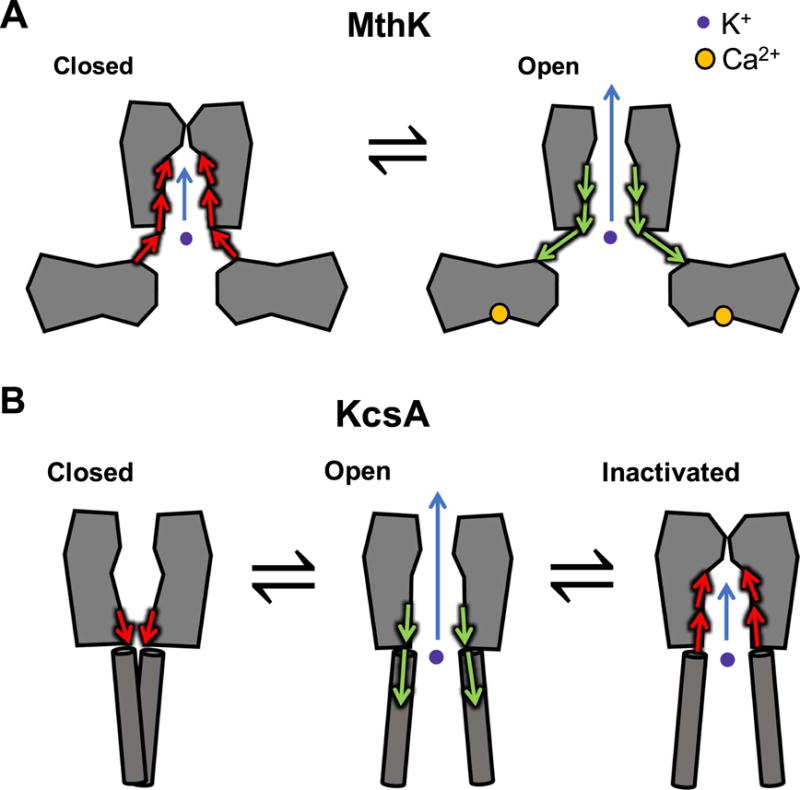
Potassium channel gating models. (A) Functional and computational experiments suggest that in MthK, the pore-lining helices do not form a tight bundle-crossing when channels are in the closed state; instead these helices appear to mediate a conformational change in the cytosolic domains to gate K+ permeation at the selectivity filter. (B) In KcsA, conformational changes at the bundle-crossing lead to channel opening, and these movements are coupled to closing at the selectivity filter (inactivation).
