Fig. 4 │. 5B8 suppresses EAE and engages fibrin target.
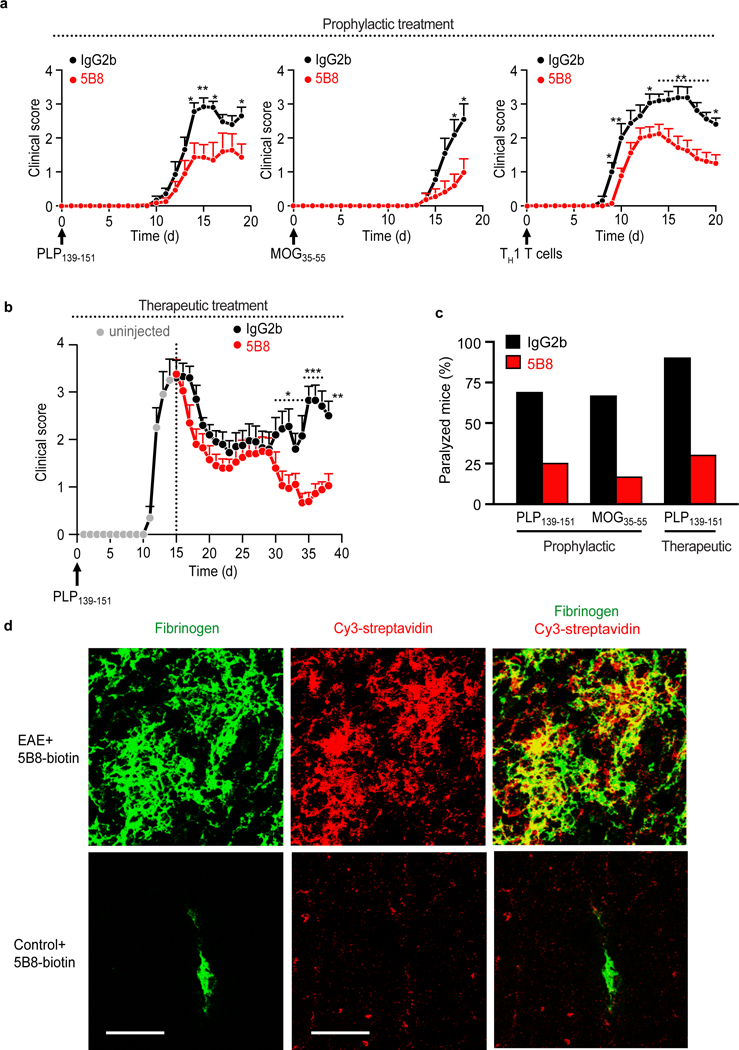
a, Reduction of clinical signs after 5B8 administration in four EAE models. Prophylactic administration of 5B8 or IgG2b isotype control in PLP139–151, MOG35–55, and adoptive transfer TH1 EAE (* P < 0.05, ** P < 0.01, linear mixed effects model with two-tailed permutation test). Mice were each given 800 μg of either 5B8 or isotype-control IgG2b every two days from day 0. Data are mean ± s.e.m. from PLP139–151 (n = 16 IgG2b and n = 16 5B8), MOG35–55 (n = 11 IgG2b and n = 12 5B8), and TH1 EAE (n = 15 IgG2b and n = 24 5B8) b, Therapeutic administration of 5B8 or IgG2b isotype control in PLP139–151 EAE after peak of disease (* P < 0.05, ** P < 0.01, *** P < 0.001, linear mixed effects model with two-tailed permutation test). For therapeutic treatment, antibodies were injected every two days starting at the peak of the initial paralytic episode. Data are mean ± s.e.m. from PLP139–151 EAE (n = 10 IgG2b and n = 10 5B8). c, Effect of 5B8 treatment on the percentage of paralyzed mice (defined as partial or complete hindlimb paralysis, score >2.5) in three EAE groups. d, Target engagement of i.p. injected biotinylated 5B8 in MOG35–55 EAE mice and healthy non-immunized control mice. Confocal microscopy of spinal cord sections from MOG35–55 EAE mice shows the spatial correlation (yellow) between biotinylated 5B8, detected with Cy3-streptavidin (red), and fibrin deposition, detected with a fibrin(ogen) antibody (green), in MOG35–55 EAE mice. Representative images are shown from n = 3 mice per group. Scale bar, 200 μm.
