Figure 2. Structural changes in the μOR stabilized by nucleotide-free Gi.
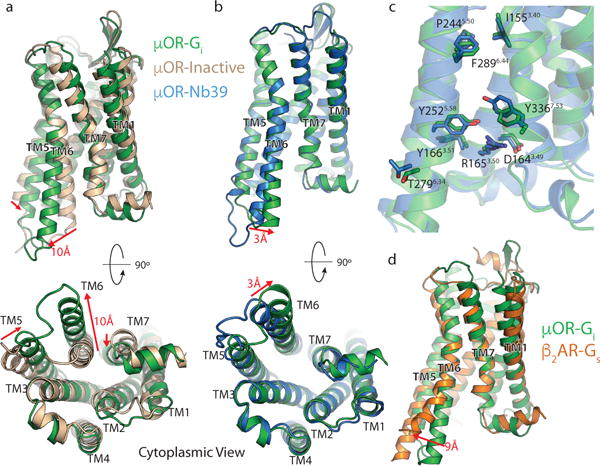
a, Comparison of inactive μOR (brown) and the Gi stabilized active state of μOR (green). b, Comparison of Nb39 and Gi stabilized active states of the μOR (blue and green, respectively). The structures are nearly identical except for a slight shift of TM6 towards TM7 in the Gi -bound state. c, Residues important for activation of the μOR show nearly identical conformations despite the difference in ligands. d, Comparison of Gs-stabilized β2AR (orange) and Gi-stabilized μOR (green). While most transmembrane helices align well between the two receptors, TM6 is kinked further outward by 9Å in the β2AR. Distance calculated between Cα of residue 6.29 (Ballesteros-Weinstein numbering) in TM6.
