Figure 4. Comparison of the receptor-G protein binding interfaces of the μOR-Gi and β2AR-Gs complexes.
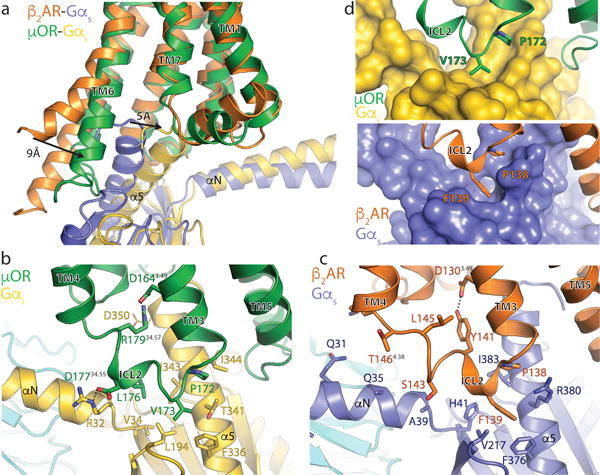
a, Comparison of the conformation of the α5 helix of Gα and receptor TM6 in β2AR-Gs and μOR-Gi complexes after alignment on the receptor. b, Interactions between ICL2 of the μOR (green) and Gαi (gold). Asp 350 of Gαi is depicted with narrow lines to indicate uncertainty in its conformation due to poor cryo-EM density for its side chain. c, Interactions between ICL2 of the β2AR (orange) and Gαs (blue). d, Surface view of the hydrophobic pockets in Gαi (top panel) and Gαs (bottom panel) that interact with a non-polar amino acid in ICL2 of the μOR and β2AR, respectively.
