Figure 7.
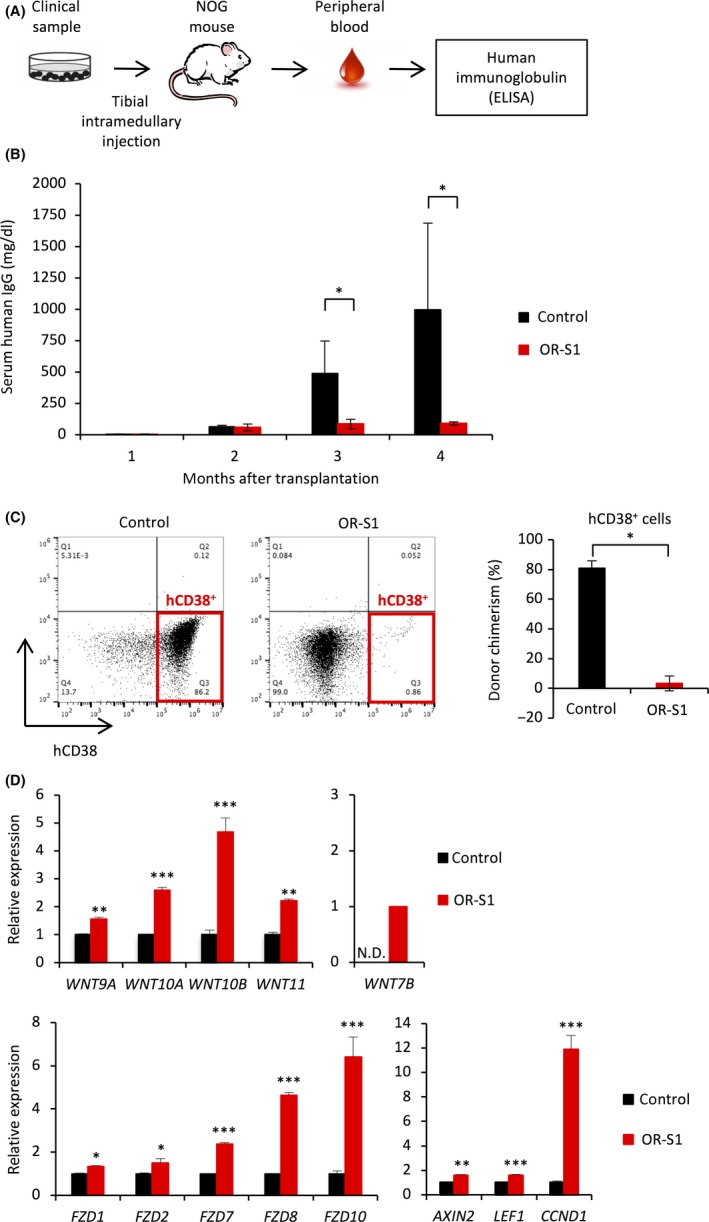
Translational implication of enhancer of zeste homolog 1/2 (EZH1/2) dual inhibition in multiple myeloma (MM). A, Experimental schema showing how successful engraftment was confirmed. Levels of human immunoglobulins in the serum of mice were measured 1 mo after intramedullary injection of clinical samples. NOG, NOD.Cg‐Prkdcscid Il2rgtm1Sug/Jic (Central Institute for Experimental Animals, Kawasaki, Japan). B, Bar graph showing the levels of human IgG in the serum of control and OR‐S1‐treated engrafted mice. Treatment with OR‐S1 (400 mg/kg per day orally for 60 d) was started 2 mo after transplantation, and levels of human IgG were monitored monthly (n = 4 per group). Error bars represent the mean ± SD. *P < .05. C, Representative FACS plots and a bar graph showing the percentage of hCD38+ cells within the bone marrow of control and OR‐S1‐treated mice with patient‐derived xenograft (PDX) at the final follow up. Error bars represent the mean ± SD. *P < .00001 (Student's t test). D, RT‐PCR analysis showing relative expression of WNT‐related genes in myeloma cells in a PDX model. Mice were given 400 mg/kg per day OR‐S1 orally for 3 wk following engraftment of tumors. Y‐axis represents the fold‐change in gene expression after normalization to that of ACTB. Error bars represent the mean ± SD. *P < .05; **P < .01; ***P < .001 (Student's t test)
