Fig. 1. AaTCP14 protein interacts with AaORA.
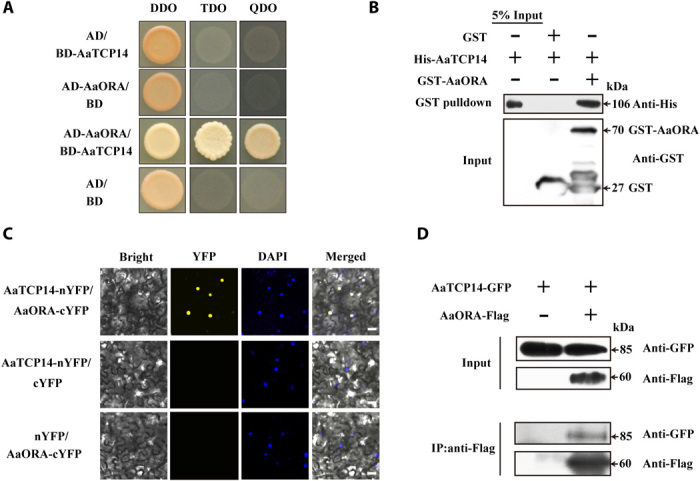
(A) Y2H analysis of AaTCP14 interaction with AaORA. Yeast cells transformed with different combinations of constructs containing AaTCP14 fused with the DNA binding domain (BD-AaTCP14), AaORA fused with the activation domain (AD-AaORA), the BD alone, and the AD alone were grown on two different selective media, SD/-Trp/-Leu/-His (TDO) and SD/-Trp/-Leu/-His/-Ade (QDO), and the control medium SD/-Trp/-Leu (DDO). Pictures were taken after 4 days of incubation at 30°C. Y2H assays were repeated three times, and representative results are shown. (B) In vitro pulldown assays of AaTCP14 and AaORA recombinant proteins. His-AaTCP14 proteins were pulled down with GST-AaORA and further detected on Western blots probed with anti-His antibody. Experiments were carried out three times, and representative results are shown. (C) Bimolecular fluorescence complementation (BiFC) analysis of the interaction between AaTCP14 and AaORA in N. benthamiana cells. AaTCP14 was fused to the N-terminal fragment of yellow fluorescent protein (AaTCP14-nYFP), and AaORA was fused to the C-terminal fragment of YFP (AaORA-cYFP). Colocalization of reconstituted YFP and nuclei was determined by 4′,6-diamidino-2-phenylindole (DAPI) staining. Three independent transfection experiments were performed. Scale bars, 20 μm. (D) Co-IP studies of AaTCP14 and AaORA complex formation in N. benthamiana leaves. Total protein extracts from N. benthamiana leaves infiltrated with constructs harboring AaTCP14-GFP and AaORA-Flag were immunoprecipitated with anti-Flag antibody. The coimmunoprecipitated proteins were detected by anti-GFP antibody. Experiments were repeated three times and similar results were obtained.
