Fig. 5. AaTCP14 and AaORA synergistically promote artemisinin biosynthesis, and AaORA is partly dependent on AaTCP14.
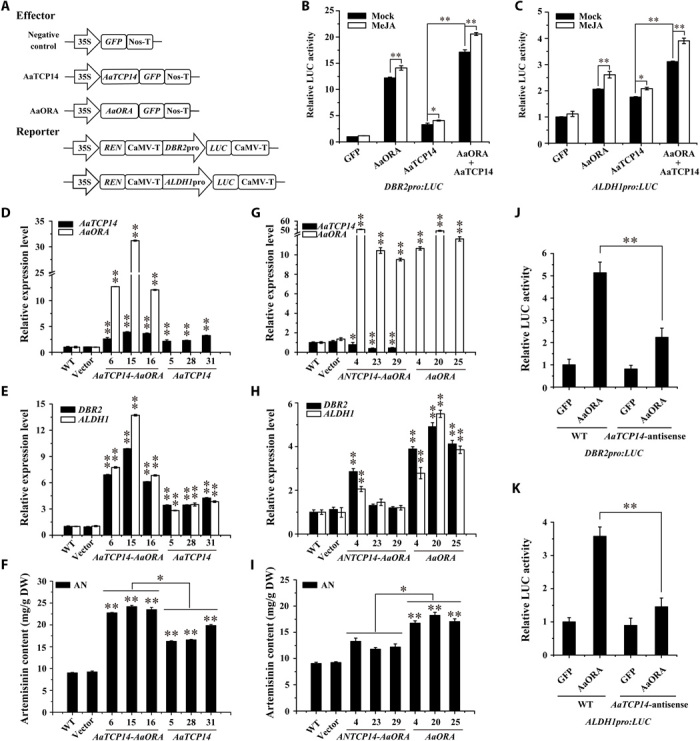
(A) A schematic representation of the constructs used in dual-LUC assays. (B and C) Activation of the DBR2 (B) and ALDH1 (C) promoters by AaORA and AaTCP14 proteins in the presence or absence of MeJA in N. benthamiana leaves. The GFP effector in the mock treatment served as a negative control, and the LUC/REN ratios of GFP were set as 1. Three independent transfection experiments were performed. The reporter strain harboring DBR2pro:LUC or ALDH1pro:LUC was mixed with the effector strains harboring 35Spro:AaTCP14 and 35Spro:AaORA at a ratio of 1:1:1. The data represent the means ± SD of three replicates from three independent experiments. *P < 0.05, **P < 0.01, Student’s t test. (D and E) Expression levels of AaTCP14 and AaORA (D) and DBR2 and ALDH1 (E) in different A. annua plants including AaTCP14-AaORA co-overexpression (AaTCP14-AaORA), AaTCP14 overexpression lines, and plants transformed with the empty vector. Actin was used as the internal standard. WT plants served as controls. The data represent the means ± SD of three replicates from three cutting propagations. **P < 0.01, Student’s t test. (F) HPLC analysis of artemisinin (AN) in the leaves of different A. annua plants including AaTCP14-AaORA and AaTCP14 overexpression lines, plants transformed with the empty vector, and the WT control. The data represent the means ± SD of three replicates from three cutting propagations. *P < 0.05, **P < 0.01, Student’s t test. (G and H) Expression levels of AaTCP14 and AaORA (G) and DBR2 and ALDH1 (H) in different A. annua plants including the AaTCP14 antisense–AaORA overexpression (ANTCP14-AaORA), AaORA overexpression lines, and plants transformed with the empty vector. Actin was used as the internal standard. WT plants served as controls. The data represent the means ± SD from three replicates from three cutting propagations. *P < 0.05, **P < 0.01, Student’s t test. (I) HPLC analysis of artemisinin (AN) in the leaves of different A. annua plants including the ANTCP14-AaORA, AaORA overexpression lines, plants transformed with the empty vector, and the WT control. The data represent the means ± SD of three replicates from three cutting propagations. *P < 0.05, **P < 0.01, Student’s t test. (J and K) Dual-LUC experiments showing the activation of the DBR2 (J) and ALDH1 (K) promoters by AaORA in A. annua protoplasts from WT and AaTCP14 antisense (ANTCP14) lines. GFP was used as a negative control, and the LUC/REN ratios of GFP in WT A. annua were set as 1. Three independent transfection experiments were performed. The data represent the means ± SD of three independent experiments. Student’s t test, **P < 0.01.
