Figure 2. The N Terminus of SNIP1 Interacts with the N Terminus of TET2.
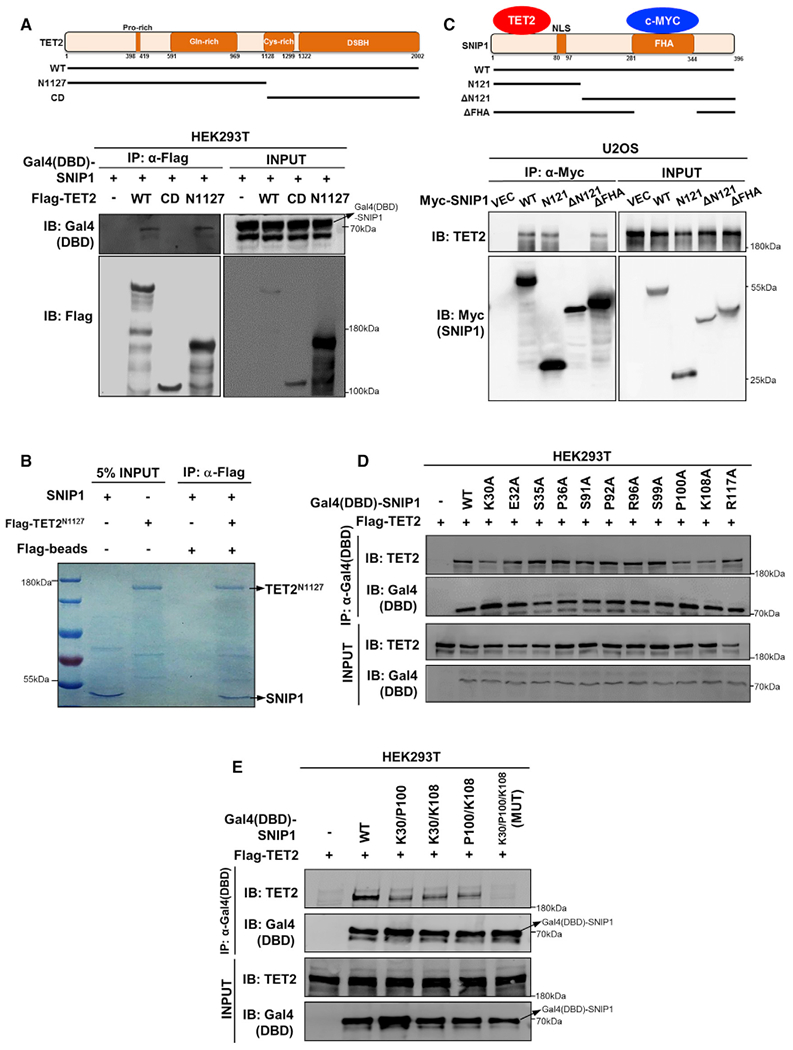
(A) SNIP1 interacts with the N-terminal domain of TET2. Schematic representation of human full-length TET2 is shown (top). Flag-tagged TET2 truncations were individually co-overexpressed with Gal4(DBD)-SNIP1 in HEK293T cells. Each truncation ofTET2 was purified by immunoprecipitation with Flag beads, followed by western blot to detect Gal4(DBD)-SNIP1 with the Gal4-DBD antibody.
(B) SNIP1 interacts with the N terminus of TET2 in vitro. Flag-tagged TET2 N terminus was overexpressed in HEK293T cells and purified by immunoprecipitation with Flag beads and subsequent Flag peptide competition. Meanwhile, GST-tagged SNIP1 was expressed in Escherichia coli and purified by immunoprecipitation with GST beads and enzyme digestion (to remove GST tag). The aforementioned purified proteins were incubated and then immunoprecipitated with Flag beads to detect the interaction of SNIP1 by Coommassie blue staining.
(C) TET2 interacts with the N-terminal domain of SNIP1. SNIP1 protein contains a nuclear localization signal domain (NLS) and a FHA domain (top). Myc-tagged truncations of SNIP1 were transiently overexpressed in U2OS cells. Each truncation of SNIP1 was purified by immunoprecipitation with a Myc antibody, followed by western blot to detect endogenous TET2.
(D and E) K30/P100/K108 residues in the N-terminal domain of SNIP1 are crucial forTET2 interaction. Gal4(DBD)-tagged wild-type or single point mutant SNIP1 was transiently co-overexpressed with Flag-TET2 in HEK293T cells. Each truncated protein of SNIP1 was purified by immunoprecipitation with the Gal4-DBD antibody, followed by western blot to detect the co-precipitated TET2 using a commercial TET2 antibody (D). Furthermore, K30/P100/K108 double- or triplemutant SNIP1 was transiently co-overexpressed with Flag-TET2 in HEK293T cells. Each wild-type or mutant SNIP1 protein was purified by immunoprecipitation with the Gal4-DBD antibody, followed by western blot to detect the co-precipitated TET2 using a commercial TET2 antibody (E). MUT, K30/P100/K108 triple-mutant SNIP1.
