Figure 6. Depletion of SNIP1 and TET2 Has No Additive Effect on Increasing DNA Double-Strand Breaks and Cell Apoptosis.
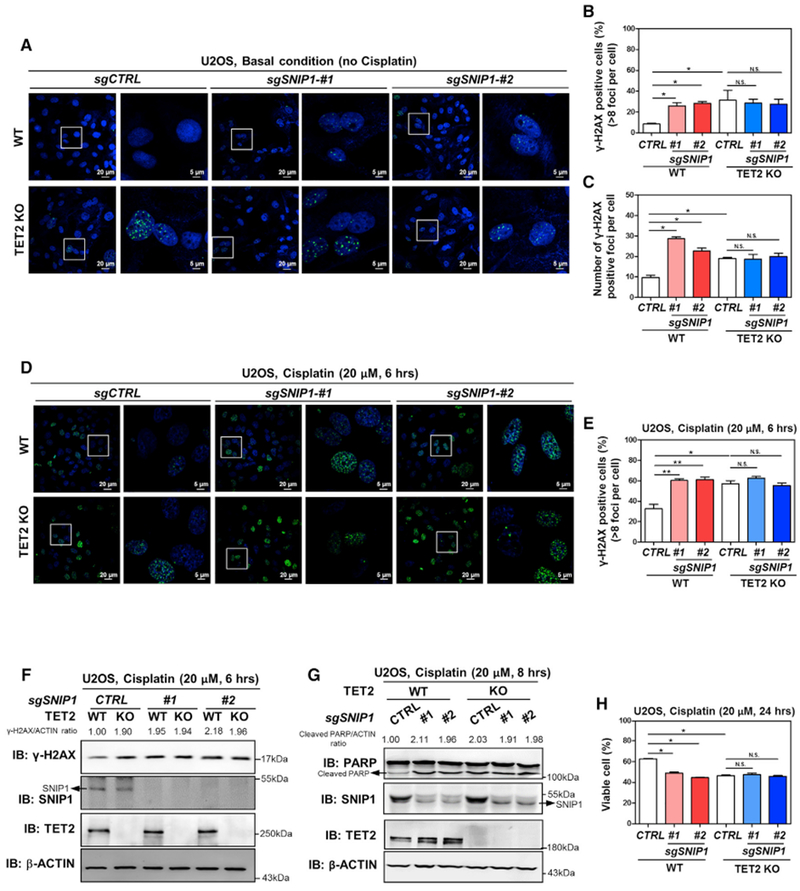
(A–C) SNIP1 knockdown and TET2 knockout have no additive effect on increasing internal DNA double-strand breaks. In the indicated U2OS stable cells, γH2AX foci was determined using immunofluorescence (A), and the percentage of γH2AX-positive cells (more than eight foci per cell) was counted from randomly selected cells of three independent experiments (B). Meanwhile, the number of γH2AX foci per cell was counted from randomly selected cells (C).
(D–F) SNIP1 knockdown and TET2 knockout have no additive effect on regulating cisplatin-induced DNA damage response. The indicated U2OS stable cells were treated with cisplatin (20 μM for 6 hr), γH2AX foci was determined using immunofluorescence (D), and the percentage of γH2AX-positive cells (more than eight foci per cell) was counted from randomly selected cells of three independent experiments (E). Additionally, γH2AX level was also determined using western blotting (F) and normalized by β-ACTIN in these U2OS stable cells.
(G and H) SNIP1 knockdown and TET2 knockout has no additive effect on regulating cisplatin-induced apoptosis. The indicated U2OS stable cells were treated with cisplatin (20 μM) as indicated, and the protein level of cleaved PARP was determined using western blotting (G), normalized by β-ACTIN. The percentage of viable cells (both PI and annexin V negative) was determined using flow cytometry (H).
Shown are average values of triplicated experiments with SD. *p < 0.05 and **p < 0.01 for the indicated comparisons. N.S., not significant.
