Figure 7. SNIP1-TET2 Interaction Plays a Crucial Role in Regulating DNA Damage Response and Cell Apoptosis.
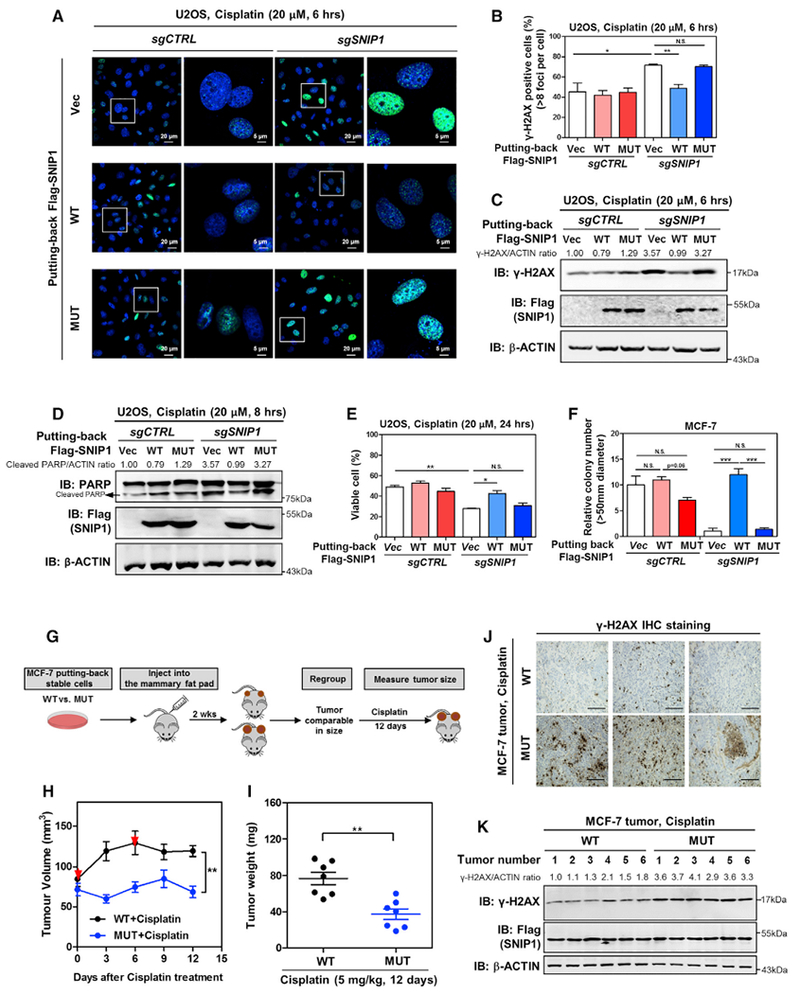
(A–C) TET2 association is required for SNIP1 to protect cells from cisplatin-induced DNA damage. The indicated U2OS stable cells were treated with cisplatin (20 μM for 6 hr), and the γH2AX level was determined by immunofluorescence (A), and the percentage of γH2AX-positive cells (more than eight foci per cell) was counted from randomly selected cells of three independent experiments (B). In addition, the protein level of γH2AX was also determined using western blotting (C), normalized by β-ACTIN.
(D and E) TET2 association is required for SNIP1 to protect cells from cisplatin-induced apoptosis. The indicated U2OS stable cells were treated with cisplatin (20 μM) as indicated, and the protein level of cleaved PARP was determined using western blotting (D), normalized by β-ACTIN. The percentage of viable cells (both PI and annexin V negative) was determined using flow cytometry (E).
(F) TET2 association is required for SNIP1 to promote anchorage-independent cell growth. The ability of indicated MCF-7 stable cells to exhibit anchorage-independent growth was determined by soft-agar colony formation assay as described in STAR Methods. Note that cells were maintained under basal condition (without cisplatin).
(G) Schematic representation of the orthotopic xenograft experiment. In brief, SNIP1-knockdown MCF-7 cells expressing wild-type or TET2-binding defective mutant SNIP1 were injected into the mammary pat of nude mice (female, 4–6 weeks old). At 2 weeks after injection, tumor-bearing nude mice with similar tumor volumes (73.69 ± 15.14 mm3 [n = 10] and 68.68 ± 14.04 mm3 [n = 10] for tumors expressing wild-type SNIP1 and TET2-binding defective mutant SNIP1, respectively) were selected and then subjected to intraperitoneal injection with cisplatin (5 mg/kg for every 6 days). The tumor volume was monitored for 12 days, and tumor weight was determined at sacrifice.
(H and I) SNIP1-TET2 interaction is vital for cisplatin sensitivity of breast cancer in vivo. The orthotopic xenograft experiment was performed as described in (G), and the tumor volume (H) and tumor weight (I) were measured at the indicated time points. Red arrows indicate cisplatin injection.
(J and K) SNIP1-TET2 interaction isvital for regulating cisplatin-induced DNA damage response in vivo. The level of γH2AX in tumor samples was detected using IHC staining (J). Representative IHC images (original magnification, 200×; scale bar, 50 μm) are shown. Meanwhile, the γH2AX level in tumor samples was also determined using western blotting (K), normalized by β-ACTIN.
Shown are average values of triplicated results with SD. *p < 0.05, **p < 0.01, and ***p < 0.001 for the indicated comparisons. N.S., not significant.
