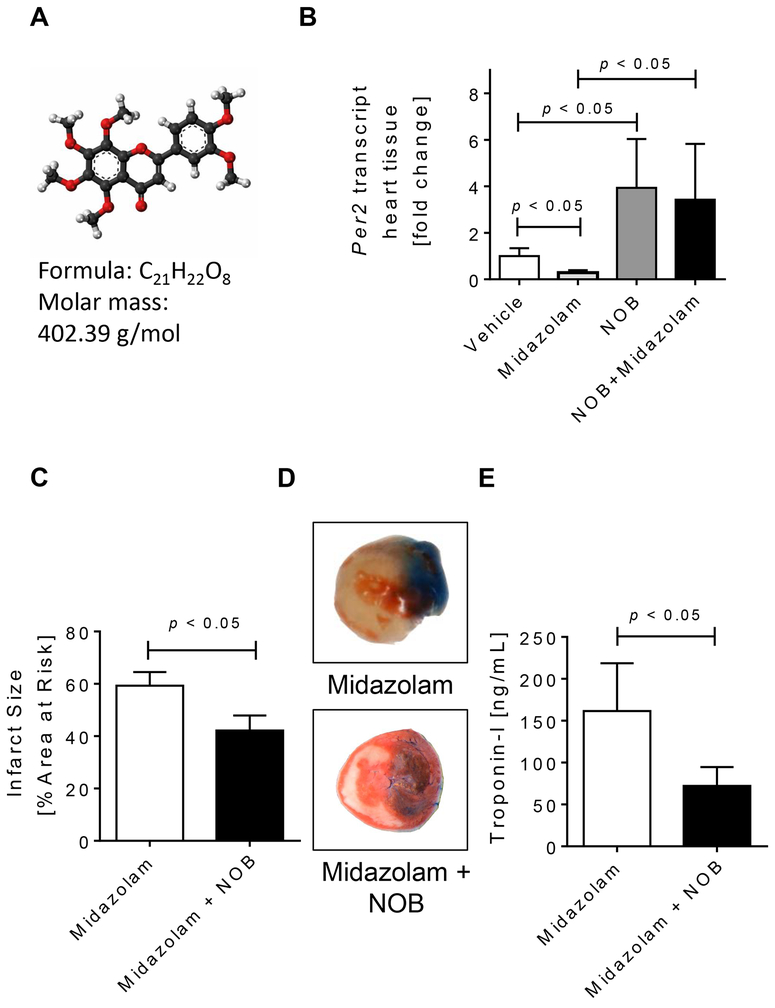
Figure 3. Nobiletin reverses the deleterious effects of midazolam.
Mice were treated i.p. with vehicle (solutol in 0.9% NaCl [ratio 1:100]), nobiletin in solutol:0.9%NaCl (ratio 1:100; 1mg/kg), midazolam (200mg/kg) or midazolam + nobiletin. Two hours later cardiac Per2 mRNA expression levels were analyzed, or mice underwent myocardial ischemia. Myocardial ischemia consisted of 60 min of ischemia followed by 120 minutes of reperfusion. Infarct sizes were measured by double staining with Evan’s blue and triphenyl-tetrazolium chloride. Infarct sizes are expressed as the percent of the area at risk (AAR) that underwent infarction. Serum troponin I concentrations were measured by enzyme-linked immunosorbent assay (ELISA). (A) Molecular formula of nobiletin, a potent PER2 enhancer. (B) Mouse cardiac Per2 mRNA levels two hours after exposure to vehicle, midazolam, nobiletin or midazolam+nobiletin. (C) Infarct sizes as the percent of AAR; (D) Representative infarct staining; (E) Serum troponin I concentrations; (n=5–7; mean±SD; p<0.05; NOB=nobiletin).