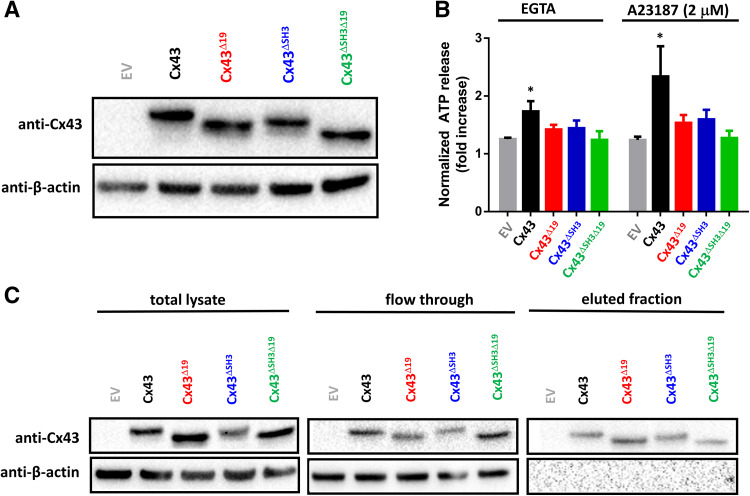
Fig. 5.
Cx43 requires the last 19 amino acids and the SH3-binding domain to enable hemichannel activity. a An immunoblot stained with anti-Cx43 (N-term) (upper blot) or anti-β-actin (lower blot) for lysates (10 µg of lysate/lane) obtained from HeLa cells transfected with empty vector (EV), Cx43, Cx43∆19, Cx43∆SH3 and Cx43∆SH3∆19 [pcDNA3.1-(−) plasmids]. b ATP release in response to EGTA or A23187 (2 µM) through hemichannels established by Cx43, Cx43∆19, Cx43∆SH3 and Cx43∆SH3∆19, transiently expressed in HeLa cells; empty vector was used as a control. ATP release is shown as fold increase compared to baseline values (n = 5). c Cell-surface biotinylation and subsequent streptavidin purification experiment performed on HeLa cells expressing empty vector, Cx43, Cx43∆19, Cx43∆SH3 and Cx43∆SH3∆19. The upper blots show Cx43 (anti-Cx43 N-term) and the lower blots show actin (anti-β-actin), an intracellular protein that serves on the one hand as a loading control and on the other hand as a negative control for the experiment. On the left, blots containing total lysates are displayed. In the middle, blots containing the flow-through fraction, which is the fraction that is not biotinylated (i.e., fraction that is not present on the surface). On the right, blots containing the eluted fraction are displayed, which is the biotinylated fraction (i.e., fraction that is present on the surface). Cx43 and the different Cx43-deletion variants appear in the surface-exposed fraction, while actin, an intracellular protein, is not, showing the validity of this approach