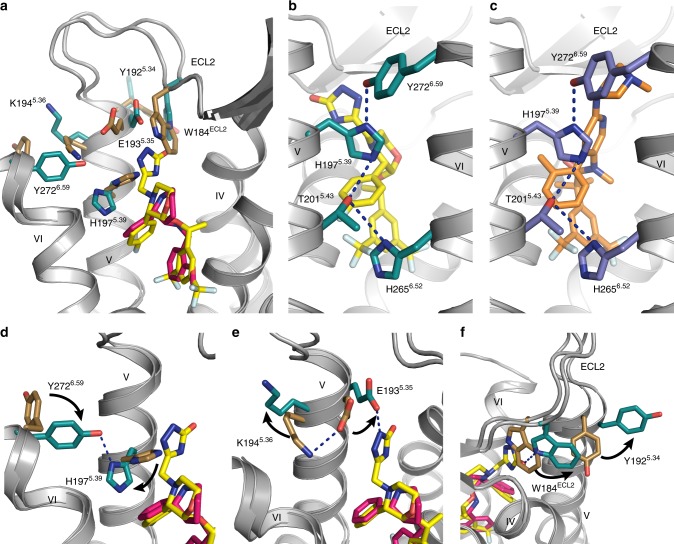
Fig. 3.
Conformational changes in NK1R induced by clinically used antagonists. a Superimposition of the CP-99,994- and aprepitant-bound NK1R structures, viewed from helix I. Residues of NK1R with side-chain orientations differing between the two receptor structures as well as the antagonists are depicted as sticks, coloured as in Fig. 1. b, c Hydrogen bond network connecting the extracellular ends of helices V and VI in the aprepitant- (b) and netupitant-bound (c) NK1R structures as viewed from the membrane plane. Hydrogen bonds are indicated as dashed blue lines. d–f Close-up views on residues with differing side-chain orientation in the CP-99,994- and the aprepitant-bound NK1R structure. Side-chain rearrangements from the CP-99,994- to the aprepitant-bound conformation are indicated by black arrows