Table 2.
Inhibition Data (IC50 values) for Cell-based Assays of Diaryl Hydroxylamines with Ring Substitutiona
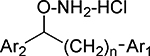 | ||||
|---|---|---|---|---|
| Compound no. | Structurea | IDO1 (μM)b | IDO2 (μM) | TDO (μM) |
| Epacadostat (INCB24360) | 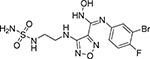 |
0.07 | 18.9 | 25.2 |
| PF-06840003 | 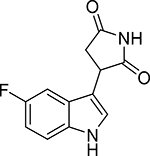 |
0.21 | 327 | 378 |
| 1 | 3 -bromo | 0.590 | 11.8 | 7.15 |
| 2 | 3,5-dichloro | 0.870 | 8.66 | 5.70 |
| 3 | 3,4-dichloro | 1.91 | 19.5 | 8.11 |
| 4 | 4,4’-dichloro | 1.09 | 12.5 | 4.53 |
| 5 | 1.43 | 36.1 | 123 | |
| 6 | 2,4-dichloro | 1.99 | 7.68 | 4.82 |
| 7 | 3,5-difluoro | 0.357 | 8.75 | 3.48 |
| 8 | 4-chloro | 7.15 | 27.8 | 23.7 |
| 9 | 3-fluoro | 0.939 | 9.99 | 6.21 |
| 10 | 2-fluoro-3 -trifluoromethyl | 0.933 | 16.8 | 5.62 |
| 11 | 6.74 | 20.5 | 58.7 | |
| 12 | 2,4-difluoro | 1.21 | 18.9 | 6.37 |
| 13 | 2 -fluoro-5 -trifluoromethyl | 1.09 | 8.94 | 9.02 |
| 14 | 2,3-dichloro | 1.87 | 8.95 | 4.54 |
| 15 | 4,4’-difluoro | 1.68 | 12.1 | 5.15 |
| 16 | 2-fluoro | 1.26 | 30.5 | 3.92 |
| 17 | 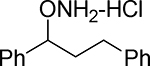 |
14.7 | 19.3 | 49.7 |
| 18 | 2-bromo | 1.46 | 12.3 | 22.5 |
| 19 | 2-chloro | 2.48 | 10.7 | 21.8 |
| 20 | 2-methoxy | 1.11 | 16.5 | 48.8 |
| 21 | 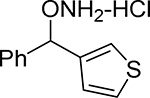 |
3.36 | 49.9 | 4.41 |
| 22 | 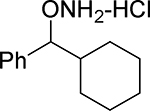 |
21.0 | 40.2 | 2770 |
| 23 | 2 -methoxy −4 -methyl −4’chloro | 1.76 | 8.77 | 19.1 |
| 24 | 3,5-bis(trifuoromethyl) | 3.35 | 14.5 | 77.4 |
| 25 | 4-hydroxy | 2.89 | 12.6 | 29.8 |
| 26 | 3 -trifluoromethyl | 1.73 | 24.7 | 13.6 |
In all structures,n=0 unless otherwise shown.
The IDO1 assay used HeLa cells, the IDO2 assay used mouse Trex cells and the TDO assay used Trex cells. Data is from a single data point except for the most potent compounds, which were done in triplicate and the results averaged.
