Abstract
The current study was carried out to evaluate the genotoxic aspects of the aqueous extracts of the Tribulus terrestris fruits by comet assay and cytogenetic procedures conditions on cultured human peripheral blood lymphocyte. After the treatment of the lymphocytes with four concentrations of the aqueous fruit extract of T. terrestris (10, 20, 40 and 80 mg/L) for 24 h it was noticed that, the presence of micronuclei and/or chromosomal aberration were monitored and a significant increase of comet cells at high concentration of T. terrestris extract 80 mg/L. Also, this study showed that the presence of micronuclei, chromosomal aberration as a chromosomal gap, fragmentation, stickiness and necrotic cells were appeared and increased with high concentrations of T. terrestris fruits extract (40–80 mg/L). On the other hand, no significant difference was observed with the low concentration of the extract (10–20 mg/L) as compared with control. The current study refers to the ability of the extract of T. terrestris fruits to do damage in the target DNA at the higher concentrations. Thus, it could be considered that the aqueous extracts of the T. terrestris fruits have genotoxic effect in the therapeutic protocols if it used in high doses.
Keywords: Comet assay, Genotoxicity, Micronuclei, T. terrestris L., Lymphocytes
1. Introduction
Tribulus L. is one of the Zygophyllaceae genera present in subtropical and desert climatic areas as Sabia, Jazan desert road. It is a silk-hairy herb with prostrate stems (Migahid, 1996, El-Sheikh et al., 2016). It is commonly distributed in India, Saudi Arabia and Yemen (Collenette, 1998). The plant was used for treatment of atherosclerosis, hypertension, and urinary tract infections (Duke et al., 2002, Trease and Evans, 2002).
T. terrestris was found to have a high concentrations of saponins, flavonoids, glycosides, alkaloids, and tannins (Usman et al., 2007). The isolated saponins was proved to induce a dose-dependent rises in nonspecific immune response (Tilwari et al., 2011), lowered blood glucose level (Li et al., 2002), decreased blood triglycerides and cholesterol levels via inhibited gluconeogenesis (Li et al., 2001, Amin et al., 2006). Additionally, T. terrestris was used as astringent, aphrodisiac, tonic, stomachic, antihypertensive, and urinary tract affections (Al-Ali et al., 2003, Chhatre et al., 2012). Other studies reported that the fruits extracts of T. terrestris was involved in the treatment of edema, abdominal distention, gonadal dysfunctions, diuretic and mild laxative (Khare, 2007, Rajendar et al., 2011, Singh et al., 2012).
Comet assay was commonly used in toxicological studies of DNA damage and known as single-cell gel electrophoresis (SCGE) (Singh et al., 1988, Olive and Banáth, 2006). Carcinogenicity and mutagenicity studies (Tice et al., 2000, Olive and Banáth, 2006). In vitro, cyto-genotoxic tests including mitotic index (MI), micronucleus (MNs) and chromosomal aberrations (CAs) assays are well-established for detecting genotoxicity (Kirsch-Volders, 1997). Clastogenicity and aneugenicity were studied well by using micronucleus (MNs) and chromosomal aberrations (CAs) assays (Scott et al., 1991, Galloway et al., 1987). A bio-screening system including comet assay and in vitro cyto-genotoxic tests might be able to detect a variety of genotoxic potentials of many medicinal herbs, and their mechanistic studies (Recio et al., 2010, Rothfuss et al., 2011).
Many authors have mentioned that these test models have the ability to predict mutagenic and carcinogenic agents that affect the living cells. Studies have showed the positive roles of these tests in detection of genotoxicity and toxicological studies for risk assessment (Morita et al., 1992, Kimura et al., 2013). Accordingly, studies on genotoxic effects of T. terrestris on human lymphocytes are rare. The current study aimed to clarify the genotoxicity of aqueous extract of T. terrestris on cultured human lymphocytes by battery of tests including comet assay, mitotic index (MI), micronucleus (MNs) and chromosomal aberrations (CAs).
2. Methodology
2.1. Plant and aqueous extract preparation
Fruits of T. terrestris were collected from natural habitats, Jazan, in the south of Saudi Arabia, which was described by Migahid (1996) during March–May 2015. The plants were identified by the plant taxonomist at the Herbarium of the Faculty of Science, Umm Al-Qura University, Saudi Arabia.
Aqueous extract of T. terrestris fruits was prepared according to Maghrani et al. (2005) under sterile conditions, the fruits were cleaned and rinsed well with water, then dipped in liquid nitrogen for drying then crushed to get the powder. The concentrations requiring were prepared in sterile distilled water, then left for 24 h at room temperature with mild shaking at regular intervals, then filtered through a Millipore filter (0.22 µm) and preserved at 4 °C in a sterile bottle until used. Different serial concentrations of the aqueous extract of T. terrestris were prepared (10, 20, 40 and 80 mg/L).
2.2. Chemicals
The materials which are used in this study were obtained from Sigma Co. (Sigma–Aldrich Company). While, the RPMI-1640 culture medium was obtained from Glaxo Laboratories Ltd.
2.3. Blood collecting and lymphocytes culturing
Human peripheral lymphocytes was obtained from fresh blood samples. Blood was obtained via venipuncture into tubes containing heparin anticoagulant from male 26 years, non-smoking healthy donor. Lymphocyte cultures were carried out according to Baeshen et al. (2009). For comet assay and cytological tests, after culturing of human lymphocytes were stimulated with phytohemagglutinin (10 µl PHA/ml), 0.5 ml of incubated blood samples (37°) was added to 4.5 ml of complete medium (RPMI-78%) plus of fetal calf serum (20%), phytohemagglutinin (2%) and 100 µg/ml for each penicillin and streptomycin. The different concentrations of prepared extracts of T. terrestris fruit were added to the test groups; meanwhile, distilled water was added to the control culture. Caps tightly screwed onto tubes and incubated at 37 °C and 5% CO2 for up to 48 h.
2.4. Slide preparation for cyto-genotoxic tests
Slide preparation was carried out according to (Baeshen et al., 2009, Zijno et al., 2007). Slides were stained with 10% Giemsa stain (10 mg Giemsa stain powder dissolved in 80 ml phosphate buffer solution, (pH 6.8), filtered and stored in a dark bottle) for 20 min. Then the slides were briefly washed in distilled water three times and kept in a dry place up to microscopic examinations.
2.5. Comet assay (SCGE)
The SCGE assay was performed 48 h after the start of incubation according to protocol of Tice et al. (2000), which is based on the original work of Singh et al. (1988), with some modifications by Klaude et al. (1996). Cultured human lymphocytes were isolated for comet assay using the procedure described by Green et al. (1992). All steps included were performed under yellow light to prevent additional DNA damage. Slides were stained with 80 µl of ethidium bromide (200 µg/ml) and cover-slipped. A total of 300 cells per treatment were examined by fluorescence microscope at 40×, with a 420–490 nm excitation filter and a 520 nm emission filter. DNA fragmentation was classified according to the method of Kobayashi et al. (1995). Apoptotic cells were concluded to form comets with large fan-like tails and small heads, while necrotic cells were concluded to form comets with relatively large heads and narrow tails of varying length (Olive et al., 1993).
2.6. Statistical analysis
Data were analyzed using analysis of variance (ANOVA) and mean separated using Tukey’s test. Score of DNA damage was calculated according to sort of Tice et al. (2000) for each sample by multiplying the number of cells in each class by the damage class, according to the formula: Total score = (0 × n0) + (1 × n1) + (2 × n2) + (3 × n3), where (n) is a number of cells in each class. Thus, the total score could range from 0 to 300.
3. Results
3.1. Effects of Tribulus terrestris fruit aqueous extract on mitotic division phases
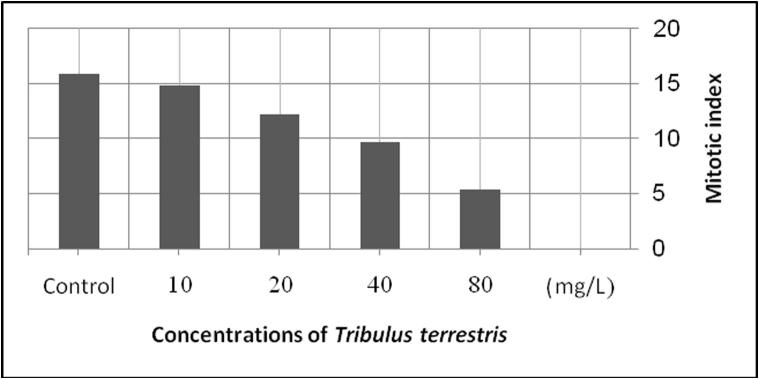
The data illustrated in Table 1 showed the effect of different concentrations of aqueous extracts of T. terrestris fruits (10, 20, 40 and 80 mg/L) against different cell division stages (prophase, metaphase, anaphase and telophase) of human blood lymphocytes treated in vitro for 24 h. It was found that the effect of 10, 20, 40 and 80 mg/L of aqueous extracts of T. terrestris fruits on mitotic cell division stages; prophase, metaphase, anaphase and telophase ranged from 1.57–6.4, 1.86–2.86, 0.78–2.78 and 1.17–3.02%, respectively. Thus, the results indicated that there is a significant reduction in the division stages of human lymphocytes when it was objected to different concentration (40–80) mg/L. On the other hand, the low concentrations of the extract (10–20) mg/L showed no significant effects on the treated lymphocytes compared with the control. Generally, it was found that the reduction in cell division is inversely proportional to the increase in the concentrations of the plant extract; high concentration of the aqueous extracts of the T. terrestris fruits caused a high significant reduction in mitotic division evidence and vice versa compared with the control (Fig. 1).
Table 1.
Percentages of mitotic division phases in human lymphocytes treated with different concentrations of aqueous extract of Tribulus terrestris fruits for 24 h.
| Extract concentration (mg/L) | Mean of mitotic division phases % |
Mean of mitotic cell division % (mitotic index) | |||
|---|---|---|---|---|---|
| Prophase | Metaphase | Anaphase | Telophase | ||
| 20 | 6.0b | 2.86b | 1.67b | 2.66b | 12.22c |
| 40 | 3.2c | 2.3c | 1.2c | 2.5b | 9.70d |
| 80 | 1.57d | 1.86d | 0.78d | 1.17c | 5.38e |
| Control | 6.57a | 3.33a | 2.84a | 3.14a | 15.88a |
| LSD | 0.1801 | 0.1669 | 0.1659 | 0.1759 | 0.1861 |
Data within the same columns followed by a same letter do not deferent significantly (Tukey‘s test, P < 0.05), e: very high significant, d: Highly significant and c: Significant, while (a & b) not significant. LSD: (least significant difference).
Fig. 1.
Mitotic index in human lymphocytes after treatment with different concentrations of aqueous extract of the fruits of the Tribulus terrestris for 24 h.
3.2. Chromosomal aberrations and nucleolus alterations
The data illustrated in Table 2 showed the chromosomal aberrations (CAs) including (c-metaphase, chromosomal gap, chromosomal fragments, chromatin bridge and stickiness) and micronucleus (MNs), bi-nucleus and tri-nucleus in addition to cell necrosis in lymphocytes after treatment with T. terrestris extract (10, 20, 40 and 80 mg/L) for 24 h. It was found that the CAs are c-metaphase, chromosomal gap, chromosomal fragments, chromatin bridge and stickiness ranged from 0.2–1.8, 0.0–1.0, 0.50–3.7, 0.2–0.5 and 0.2–3.0%, respectively.
Table 2.
Chromosomal aberration, nucleus abnormalities, and cell necrosis in human lymphocytes treated with different concentrations of aqueous Tribulus terrestris fruit extract for 24 h.
| Concentration of the extract (mg/L) | Chromosomal aberration% |
Micronucleus % | Bi-nucleate % | Tri-nucleate % | Necrosis cells% | Mean of total of abnormalities% | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||||
| 10 | 0.20 | 0.00 | 0.50 | 0.20 | 0.90 | 0.20 | 0.30 | 0.00 | 0.00 | 2.1d |
| 20 | 0.30 | 0.10 | 1.00 | 0.30 | 1.00 | 0.30 | 0.80 | 0.10 | 0.00 | 4.1c |
| 40 | 1.40 | 0.60 | 2.50 | 0.40 | 2.40 | 2.90 | 1.40 | 0.50 | 0.8 | 12.9b |
| 80 | 1.80 | 1.00 | 3.70 | 0.50 | 3.00 | 3.70 | 2.10 | 0.40 | 1.3 | 17.5a |
| Control | 0.10 | 0.00 | 0.20 | 0.00 | 0.20 | 0.10 | 0.20 | 0.00 | 0.00 | 0.8e |
| LSD | 0.5578 | |||||||||
Data within the same columns followed by the same letter do not differ significantly (Tukey‘s test, P < 0.05), 1: c-metaphase 2: chromosomal gap 3: chromosomal fragments 4: chromatin bridge 5-stickiness.
On the other hand, nucleolus alterations %; MNs, bi-nucleus and tri-nucleus ranged from 0.2–3.7, 0.3–2.1 and 0.0–0.4, respectively, while the cell necrosis ranged from 0.0 to 1.3. Generally, the results showed that a significant difference in establishment of MNs, bi-nucleate and tri-nucleate, especially after the high concentration (40–80 mg/L) treatment, compared with the control.
Also, it was noticed that the formation of CAs which includes a chromosomal gap, chromosomal fragments, chromatin bridge, stickiness, c-metaphase in addition to necrotic cells also were observed after treatment with a high concentration (40–80 mg/L) compared with the control. Thus, when we compare the mean values of different cells in each treatment with that of the control, it was noticed that there is a high significant difference in the general nucleus alterations as well as a CAs compared to the control.
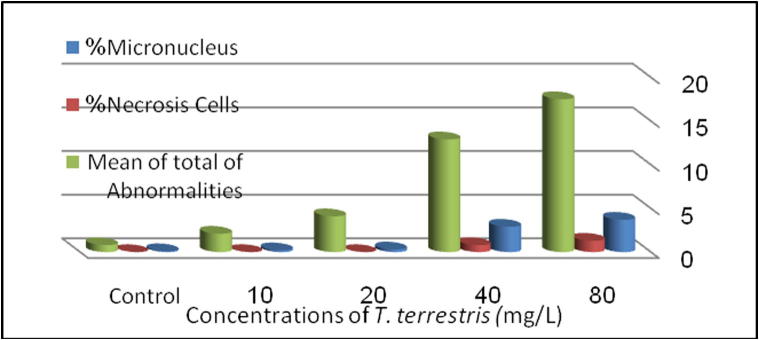
Also, it was noticed that there is a direct correlation between MNs percentages, CAs percentages and the concentration of the plant extract as a dose-dependent (Fig. 1).
3.3. DNA damage assessment
The results obtained in the Table 3 showed the effects of different concentration of T. terrestris fruits extract (10, 20, 40 and 80 mg/L) on genomic DNA of human lymphocyte cultured. Results showed that the damaged cells were increased at higher concentrations (40 and 80 mg/L) and decreased at lower concentrations (10 and 20 mg/L). Also, it was found that the most common grades were (1 and 2), while the last one were graded (3). So, it was found that there is a direct correlation between the concentrations of the T. terrestris fruit extract which is used in this experiment (10, 20,40,80 mg/L) and the DNA damaged% (Fig. 2).
Table 3.
Assessment of human lymphocytes damage after treatment with different concentration of aqueous T. terrestris fruit extract.
| Concentration of the extract (mg/L) | Classification of comet cells % |
Mean of total cell damage cells % | Mean of DNA damage score % | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| 10 | 93.5 | 2.4 | 4.1 | 0.0 | 6.5d | 10.6d |
| 20 | 87.6 | 6.0 | 4.3 | 2.1 | 12.4c | 20.9c |
| 40 | 40.0 | 24.3 | 11.0 | 6.0 | 41.0b | 64.3b |
| 80 | 27.0 | 21.2 | 16.0 | 8.0 | 45.5a | 77.2a |
| Control | 96.3 | 1.1 | 1.3 | 0.0 | 2.4e | 3.7e |
| LSD | 1.0608 | 1.9592 | ||||
0: undamaged DNA 1: Tail length was equal to the head diameter (slight DNA damage), 2: Tail length was twice of the head diameter (moderate DNA damage), 3: Tail length more than twice head diameter (severe DNA damage). Data within the same columns followed by the same letter do not differ significantly (Tukey‘s test, P < 0.05).
Fig. 2.
Micronucleus, cell necrosis and total abnormalities in human lymphocytes treated with different concentrations of aqueous Tribulus terrestris fruit extract for 24 h.
4. Discussion
The present study illustrated genotoxic effects of different concentrations of the T. terrestris fruits extract on cultured human lymphocytes. A significant decrease in cell division phases at higher concentration (40–80) mg/L was recorded as compared to control. The decrease in cell division phases may be due to the suppression of DNA synthesis at prophase and metaphase stages or high concentrations of the extract components may be linked to DNA across the peripheral hydroxyl groups which lead to the suppression of DNA synthesis. These results are going in line with (Ochi et al., 1987, Snyder, 1988) who demonstrated that the main effective stage in the cell cycle division was between prophase and metaphase. Also, the effect may be caused by a defect in the biosynthesis of the DNA or RNA or protein process leading to reduced formation of nucleic acids and proteins (Romani et al., 2015). Also, Giri et al., 1984, Dhir et al., 1984 concluded that the cationic derivatives, which separated from the plant extract may be linked to the terminal phosphate groups in DNA by the way chelate complex was formed which are impeding the DNA replication progress in cell division cycle (Qari, 2008a, Qari, 2010). On the other hand, the low concentration treatment (10–20) mg/L showed low significant differences in cell division phases which may be indicated that the role of the concentration factor that may influence the division of lymphocyte, because these treatments represent low concentrations of all active ingredients. So, results emphasize a dose-dependent effects for the different concentrations and the negative genetic influences.
In addition, the study revealed a high percentages of nucleolus alterations associated with many CAs in case of high concentrations (40–80 mg/L) of the extract and it was significantly different as compared with the control group. There is no doubt that such results indicated that one or more components of the aqueous extract of the fruits are able to cause chromosomal aberrations during the cell cycle, which might be leading to the formation of micronuclei. The occurrence of micronuclei was increased significantly after treatment with high concentrations of the extract (40 and 80 mg/L) reflecting the increased amount of DNA damage in case of increasing the therapeutic dose. These results agree well with that of Qari (2008b) and Morais et al. (2016) who mentioned that these types of chromosomal aberration may be due to genetic or environmental factors. Also, Amer and Ali (1983) concluded that the stickiness aberrations may be the result of the impact on the spindle filaments. These results also are going in line with that of Sarbhoy et al. (1991) and Zickler and Kleckner (1999) who suggested that chromosomal stickiness might be produced by one or more component of the extract leading to changing link regions of chromosome proteins (nucleic acids) or due to inhibition of metaphase stage of cell division.
On the other hand, the cells necrosis occurrence may be due to the direct effect of the extract on the ionic structure of DNA. These leads to changes in the ion distribution of the DNA group functions as well as disrupting DNA self-protection (Methylation) against endonuclease which may lead to DNA degradation. Whereas, it was noticed during the investigation, the presence of a lot of cells with high pigmented DNA and others without DNA as a result of DNA degradation, while the presence of highly pigmented DNA may be due to high circling of DNA strands around itself in a specific area (Bird, 2002, Shen et al., 2016).
The obtained results showed that the comet cell formation was increased after exposure to the higher concentrations (40–80 mg/L) of the extract as compared with the control, that confirm the previous results which indicated the formation of micronuclei after treatment with the extract of T. terrestris fruits. On the other hand, the results might indicated the presence of one or more component of the T. terrestris fruits extract able to break the DNA strands of DNA in a certain way, this break in the DNA may be occurring in one or more of DNA strand alternatively, which leading to chromatin gap due to deletion/absence of one or more nuclei and couldn’t be repaired by the repair mechanism. Generally, these results agree with the study of (Kim et al., 2011) who concluded that the extract of T. terrestris reduces the division rate as a result of a defect in the DNA suggesting the possibility of using of T. terrestris fruit extract as anticancer due to its ability to slow down the cell division, its safety on the DNA molecule at lower doses and its antioxidant contents (Kumar et al., 2006, Neychev et al., 2007, Stuven and Pflugmacher, 2007, Kumar et al., 2009, Kim et al., 2011).
However, Chhatre et al. (2014) suggested that the T. terrestris extract contains many components which have the therapeutic ability; saponins, flavonoids, glycosides, alkaloids, and tannins. Consequently, due to the lack of molecular studies on the impact of this plant extracts on the genetic materials, the current study suggests that one or more components of the T. terrestris extract have the affinity or ability to emerge itself and occupies a space within the nucleotides in the DNA strand. Thus, in the comet assay application, the double strand of DNA decryption occurs, the breakdown in the areas which involved the extract leading to the presence of huge amount of micronuclei and many chromosomal aberrations. In conclusion, the aqueous extracts of the T. terrestris fruits might have genotoxic effect in the therapeutic protocols if it used in a higher doses.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Ali M., Wahbi S., Twaij H., Al-Badr A. Tribulus terrestris: Preliminary study of its diuretic and contractile effects and comparison with Zea mays. J. Ethnopharmacol. 2003;85:257–260. doi: 10.1016/s0378-8741(03)00014-x. [DOI] [PubMed] [Google Scholar]
- Amer S.M., Ali E.M. Cytological effect of the insecticide dipterex “trichlorophon” on Vicia faba. Cytologia. 1983;48:761–770. [Google Scholar]
- Amin A., Lotfy M., Shafiullah M., Adeghate E. The protective effect of Tribulus terrestris in diabetes. Ann. N. Y. Acad. Sci. 2006;1084:391–401. doi: 10.1196/annals.1372.005. [DOI] [PubMed] [Google Scholar]
- Baeshen N.A., Sabir J.S.M., Abo-Aba S.E.M., Qari S.H. Evaluation of the cytogenetic status and DNA integrity of human lymphocytes after exposure to an aqueous extract of Rhazya stricta leaves in vitro. J. Appl. Sci. Res. 2009;5(8):986–994. [Google Scholar]
- Bird A. DNA methylation patterns and epigenetic memory. Gene. Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Chhatre S., Nesari T., Somani G., Kenjale R., Sathaye S. Comparative evaluation of diuretic activity of different extracts of Tribulus terrestris fruits in experimental animals. Int. J. Res. Phytochem. Pharmacol. 2012;3:129–133. [Google Scholar]
- Chhatre S., Nesari T., Somani G., Kanchan D., Sathaye S. Phytopharmacological overview of Tribulus terrestris. Pharmacogn. Rev. 2014;8(15):45–51. doi: 10.4103/0973-7847.125530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collenette S. International Asclepiad Society; 1998. Checklist of Botanical Species in Saudi Arabia. I: 80. [Google Scholar]
- Dhir H., Sharma A., Talukder G. Cytotoxic action of lead on plant systems. Acta Botanica. 1984;14:213–219. [Google Scholar]
- Duke J., Duke P.K., Cellier J.L. Duke Handbook of Medicinal Herbs. second ed. CRC Press; United States: 2002. p. 595. [Google Scholar]
- El-Sheikh T.M.Y., Al-Fifi Z.I.A., Alabboud M.A. Larvicidal and repellent effect of some Tribulus terrestris L., (Zygophyllaceae) extracts against the dengue fever mosquito, Aedes aegypti (Diptera: Culicidae) J. Saudi Chem. Soc. 2016;20:13–19. [Google Scholar]
- Galloway S.M., Armstrong M.J., Reuben C., Colman S., Brown B., Cannon C., Bloom A.D., Nakamura F., Ahmed M., Duk S., Rimpo J., Margolin G.H., Resnick M.A., Anderson G., Zeiger E. Chromosome aberration and sister chromatid exchanges in Chinese hamster ovary cells: evaluation of 108 chemicals. Environ. Mol. Mutagen. 1987;10(10):1–175. doi: 10.1002/em.2850100502. [DOI] [PubMed] [Google Scholar]
- Giri, A.K., Singh, O.P., Sharma, A., Sunal, R., Talukder, G., 1984. Comparative Effects of Chronic Treatment with Certain Metals on Cell Division Cytologia, 4, 654–665.
- Green M., Low J., Harcourt S.A., Akinluyi P., Row T., Cole J., Anstey A.V., Arlett C.V. UV-C sensitivity of unstimulated and stimulated human lymphocytes from normal and Xeroderma pigmentosum donors in the comet assay: a potential diagnostic technique. Mutat. Res. 1992;273:137–144. doi: 10.1016/0921-8777(92)90075-e. [DOI] [PubMed] [Google Scholar]
- Khare C.P.B. Springer Verlag; Heidelberg: 2007. Indian medicinal plants: an illustrated dictionary; pp. 669–671. [Google Scholar]
- Kim H.J., Kim J.C., Min J.S., Kim M.J., Kim J.A., Kor M.H. Aqueous extract ofTribulus terrestris Linn. induces cell growth arrest and apoptosis by down-regulating NF-κB signaling in liver cancer cells. J. Ethnopharmacol. 2011;136:197–203. doi: 10.1016/j.jep.2011.04.060. [DOI] [PubMed] [Google Scholar]
- Kimura A., Miyata A., Honma M.A. Combination of in vitro comet assay and micronucleus test using human lymphoblastoid TK6 cells. Mutagenesis. 2013;28(5):583–590. doi: 10.1093/mutage/get036. [DOI] [PubMed] [Google Scholar]
- Kirsch V.M. Towards a validation of the micronucleus test. Mutat. Res. 1997;392:1–4. doi: 10.1016/s0165-1218(97)00039-6. [DOI] [PubMed] [Google Scholar]
- Klaude, M., Eriksson, S.,Nygren, J., Ahnström, G., 1996.The Comet Assay: Mechanisms and Technical Considerations Mutation Research - DNA Repair. 363(2), pp. 89–96. [DOI] [PubMed]
- Kobayashi, H., Sugiyama, C., Morikawa, Y., Hayashi, M., Sofuni, T.A., 1995.Comparison Between Manual Microscopic Analysis and Computerized Image Analysis in the Single Cell Gel Electrophoresis Assay MMS Commun. 3, pp. 103–115.
- Kumar M., Soni A.K., Shukla S., Kumar A. Chemopreventive potential of Tribulus terrestris against 7, 12- dimethylbenz (a) anthracene induced skin papillomagenesis in mice. Asian Pac. J. Cancer Prev. 2006;7:289–294. [PubMed] [Google Scholar]
- Kumar, M., Panwar, M., Samarth, R., Kumar, A., 2009. Evaluation of Radiomodulatory Influence of Tribulus terrestris Root Extract Against Gamma Radiation: Hematological, Biochemical and Cytogenetic Alterations in Swiss Albino Mice, Pharmacology online. 1, pp. 1214–1228.
- Li M., Qu W., Chu S., Wang H., Tian C., Tu M. Effect of the decoction of Tribulus terrestris on mice gluconeogenesis. Zhong Yao Cai. 2001;24:586–588. [PubMed] [Google Scholar]
- Li M., Qu W., Wang Y., Wan H., Tian C. Hypoglycemic effect of saponin from Tribulus terrestris. Zhong Yao Cai. 2002;25:420–422. [PubMed] [Google Scholar]
- Maghrani M., Naoufel A.Z., Jean B.M., Eddouks M. Antihypertensive effect of Tribulus terrestris L. in spontaneously hypertensive rats. J. Ethnopharmacol. 2005;80(1-2):193–197. doi: 10.1016/j.jep.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Migahid A.M. 4th Ed. King Saud University Libraries; Riyadh, Saudi Arabia: 1996. Flora of Saudi Arabia; pp. 60–77. [Google Scholar]
- Morais, C.R., Carvalho, S.M., Araujo, G.R., Souto, H.N., Bonetti, A.M., Morelli, S., Campos, J.E.O., 2016. Assessment of Water Quality and Genotoxic Impact by Toxic Metals in Geophagus Brasiliensis Chemosphere, 152, 328–334. [DOI] [PubMed]
- Morita T., Nagaki T., Fukuda I., Okumura K. Clastogenicity of low PH to various cultured mammalian cells. Mutat. Res. 1992;268:297–305. doi: 10.1016/0027-5107(92)90235-t. [DOI] [PubMed] [Google Scholar]
- Neychev V.K., Nikolova E., Zhelev N., Mitev V.I. Saponins from Tribulus terrestris L. are less toxic for normal human fibroblasts than for many cancer lines: Influence on apoptosis and proliferation. Exp. Biol. Med. 2007;232:126–133. [PubMed] [Google Scholar]
- Ochi T., Takahahi K., Ohsawa M. Indirect evidence for the induction of a prooxidant state by cadmium chloride in cultured mammalian cells and a possible mechanism for the induction. Mutat. Res. 1987;180:257–266. doi: 10.1016/0027-5107(87)90222-3. [DOI] [PubMed] [Google Scholar]
- Olive P.L., Banáth J.P. The comet assay: a method to measure DNA damage in individual cells. Nat. Protoc. 2006;1:23–29. doi: 10.1038/nprot.2006.5. [DOI] [PubMed] [Google Scholar]
- Olive P.L., Frazer G., Banath J.P. Radiation-induced apoptosis measured in TK6 human B lymphoblast cells using the comet assay. Radiat. Res. 1993;136(1):130–136. [PubMed] [Google Scholar]
- Qari S.H. In vitro evaluation of anti-mutagenic effect of Origanum majorana on root cells of Vicia faba. J. Taibah Univer. Sci. 2008;1:6–10. [Google Scholar]
- Qari S.H. Molecular and biochemical evaluation of genetic effect of Calotropis procera (Ait.) latex on Aspergillus terreus (Thom) Indian J. Exp. Biol. 2008;46:725–730. [PubMed] [Google Scholar]
- Qari S.H. DNA-RAPD fingerprinting and cytogenetic screening of genotoxic and antigenotoxic effects of aqueous extracts of Costus speciosus (Koen.) JKAU: Sci. 2010;22(1):133–152. [Google Scholar]
- Rajendar B., Bharavi K., Rao G.S., Kishore P.V., Kumar P.R., Kumar C.S. Protective effect of an aphrodisiac herb Tribulus terrestris Linn. on cadmium-induced testicular damage. Indian J. Pharmacol. 2011;43:568–573. doi: 10.4103/0253-7613.84974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio L., Hobbs C., Caspary W., Witt K.L. Dose-response assessment of four genotoxic chemicals in a combined mouse and rat micronucleus (MN) and comet assay protocol. J. Toxicol. Sci. 2010;35:149–162. doi: 10.2131/jts.35.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani I., Manavsk N., Morosetti A., Tadini L., Maier S., Kühn K., Ruwe H., Schmitz-Linneweber C., Wanner G., Leister D., Kleine T. A member of the arabidopsis mitochondrial transcription termination factor family is required for maturation of chloroplast transfer RNAIle (GAU) Plant Physiol. 2015;169(1):627–646. doi: 10.1104/pp.15.00964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfuss A., Honma M., Czich A., Aardema M.J., Burlinson B., Galloway S. Improvement of in vivo genotoxicity assessment: combination of acute tests and integration into standard toxicity testing. Mutat. Res. 2011;723:108–120. doi: 10.1016/j.mrgentox.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Sarbhoy R.K., Sharma A., Singh R.M. The Pisum test and alternative in environmental studies; the relative toxicity of Pesticides. J. Environ. Biol. 1991;12:137–141. [Google Scholar]
- Scott D., Galloway S.M., Marshall R.R., Ishidate M., Jr., Brusick D., Ashby J., Myhr B.C. Genotoxicity under extreme culture conditions. Mutat. Res. 1991;257:147–204. doi: 10.1016/0165-1110(91)90024-p. [DOI] [PubMed] [Google Scholar]
- Shen J., Liu Y., Ren X., Gao K., Li Y., Li S., Yao J., Yang X. Changes in DNA methylation and chromatin structure of pro-inflammatory cytokines stimulated by LPS in broiler peripheral blood mononuclear cells. Poult. Sci. 2016;95(7):1636–1645. doi: 10.3382/ps/pew086. [DOI] [PubMed] [Google Scholar]
- Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Singh S., Nair V., Gupta Y.K. Evaluation of the aphrodisiac activity of Tribulus terrestris Linn. in sexually sluggish male albino rats. J. Pharmacol. Pharmacother. 2012;3:43–47. doi: 10.4103/0976-500X.92512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R.D. Role of active oxygen species in metal - induced DNA strand breakage in human diploid fibroblasts. Mutat. Res. 1988;193:237–246. doi: 10.1016/0167-8817(88)90034-x. [DOI] [PubMed] [Google Scholar]
- Stuven J., Pflugmacher S. Antioxidative stress response of Tribulus terrestris L. due to exposure to cyanobacterial secondary metabolites. Toxicon. 2007;50(1):85–93. doi: 10.1016/j.toxicon.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Tice R.R., Agurell E., Anderson D. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000;35:206–221. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Tilwari A., Shukla N.P., Devi U. Effect of five medicinal plants used in Indian system of medicines on immune function in Wistar rats. Afr. J. Biotechnol. 2011;10:16637–16645. [Google Scholar]
- Trease G.E., Evans W.C. Pharmacognosy. Fifteenth ed. Harcourt Brace and Company Asia Pvt. Ltd.; Singapore: 2002. p. 27. (A Taxonomic Approach to The Study of Medicinal Plants and Animal Derived Drugs). [Google Scholar]
- Usman H., Abdulrahman F., Ladan A. Phytochemical and antimicrobial evaluation of Tribulus terrestris L. growing in Nigeria. Res. J. Biol. Sci. 2007;2:244–247. [Google Scholar]
- Zickler D., Kleckner N. Meiotic chromosomes: integrating structure and function. Annu. Rev. Genet. 1999;33:603–754. doi: 10.1146/annurev.genet.33.1.603. [DOI] [PubMed] [Google Scholar]
- Zijno A., Saini F., Crebelli R. Suitability of cryopreserved isolated lymphocytes for the analysis of micronuclei with the cytokinesis-block method. Mutagenesis. 2007;22(5):311–315. doi: 10.1093/mutage/gem018. [DOI] [PubMed] [Google Scholar]