Abstract
Background:
Tuberculosis (TB) and diabetes are the world's leading public health issues. They are the cause of morbidity, mortality, and pose a burden on the healthcare system.
Aims and Objectives:
The aim and objective of this study were to study the prevalence of diabetes and its predictors among TB patients currently on treatment. The secondary objective was to examine the self-reported blood glucose monitoring and antidiabetic drug adherence practice among diabetic TB patients.
Methodology:
This cross-sectional study was undertaken on 275 TB cases enrolled from selected designated microscopy centers. Self-reported information on diabetes, tobacco usage, and family history of TB was collected by trained investigators. In addition, for diabetic tubercular patients, the study investigators inquired about the type of treatment taken (allopathic/traditional), frequency of getting blood sugar tested, and daily drug adherence. For comparison between the “diabetes TB comorbidity” and “TB only group,” Chi-square test of significance was used, and odds ratios were reported. Data were analyzed using Epi Info software (CDC Atlanta).
Results:
The prevalence of diabetes among TB patients was found to be 13.1% (known diabetics –9.1% and new diabetics –4.0%). There were 25.5% of current/former smokers (70/275) and 13.1% of current/former smokeless tobacco users (36/275). In logistic regression analysis, age 50 years and above emerged as a significant predictor for diabetes TB comorbidity (adjusted odds ratio = 9.8 [4.3–22.3]).
Conclusion:
Diabetes is prevalent comorbidity in TB patients. Age more than 50 years significantly increases the odds of this twin morbidity.
Keywords: Diabetes, tobacco, tuberculosis
INTRODUCTION
The World Health Organization has called upon countries of the South-East Asia Region to gear up efforts for ending tuberculosis (TB) by the year 2030. India stands committed to meet this objective and is a signatory to the “End TB Strategy.” The “Revised National TB Control Program” of India has treated millions of patients, though the rate of decline in TB cases has been slow.[1] Among the numerous reasons which adversely impact TB control in India are the urbanization and accompanying lifestyle changes which have led to a rapid increase in noncommunicable diseases.[2,3]
Studies have established the bidirectional link between diabetes and TB. It is hypothesized that diabetes worsens the clinical course of TB treatment. Furthermore, in TB, the glycemic control in diabetics is impaired.[4,5] A plausible hypothesis of TB correlation is the impaired host defense in individuals in diabetics. An immunological study conducted by Yamashiro postulated that reduced production of Th1-related cytokines and nitrous oxide in mice accounts for the hampered host defense against mycobacterium TB infection under diabetic conditions.[6] Further, the literature review shows that various risk factors, such as sociodemographic,[7] family history of TB,[8] smoking tobacco,[9] and type of TB,[7] are associated with it.
Currently, there is limited research work on diabetes mellitus (DM) in TB patients; none in the study area. In this backdrop, the current study was conducted with an objective to study the prevalence of diabetes and its predictors among TB patients currently on treatment. The secondary objective was to examine the self-reported blood glucose monitoring and antidiabetic drug adherence practice among diabetic TB patients.
METHODOLOGY
This was a cross-sectional study conducted in Chandigarh city. There are four TB units and 18 designated microscopy centers (DMC). The study participants included TB cases ≥18 years of age including new/retreatment cases, extrapulmonary cases, multidrug-resistant (MDR) cases, and those visiting DMC for taking antitubercular medicines. The sample size of 296 was calculated based on an estimated prevalence of DM in TB patients of 20% (based on a pilot study); with a precision of 5% and a nonresponse rate of 20%. For achieving this sample size, 6 DMCs were randomly selected out of 18 DMCs using random number tables. The desired sample size of 296 was then divided equally among all the six study sites. Trained field investigators visited the selected DMC based on a pre-decided schedule, and daily ten patients who came to the TB health visitor (TBHV) were consecutively enrolled in the study. In this way, field investigators kept on visiting a study site till the desired sample size was enrolled. The study participants were interviewed in private after explaining the objective of the study and obtaining written informed consent.
In the study area, TB patients are screened for diabetes as per guidelines.[10] Patients are initially screened with random blood sugar (RBS) test. If the RBS is ≥140 mg/dL, the patient fasting blood glucose is tested. A value of FBS value ≥126 mg/dL indicates diabetes. All such patients are referred to a physician for definitive diagnosis and care. The diagnosed cases of diabetes are documented in the treatment cards of the patients which are available with the TBHV of the DMC. For the current study, the study investigators questioned the study participants regarding their diabetes status (yes or no). All those study participants who told that they were newly diagnosed at the time of starting antitubercular drugs were called “new diabetics.” Those who were taking antidiabetic medicines before being diagnosed with TB were classified as “previously diagnosed diabetics.” The field investigators confirmed the diabetes status of the study participants by looking at their antidiabetic prescription records of patients and checking the documentation on treatment card available with TBHV. “Current tobacco users” were defined as those who had smoked or chewed tobacco in the last 30 days preceding the study. “Former tobacco smokers” were those who had quit smoking tobacco more than 30 days preceding the study. “Never-users” were defined as persons who had never smoked or chewed tobacco even once in their lifetime. For second-hand smoke exposure, study participants were asked whether any individual smokes in the home environment or workplace. Other independent variables included sociodemographic characteristics (age group, sex, and level of education) and family history of TB. Diabetic study participants were asked about the type of treatment taken (allopathic/traditional), frequency of getting blood sugar tested, and daily drug adherence.
Data were analyzed using Epi-info software for windows (Centre for Disease Control, Atlanta, Georgia, USA). For comparison between the “diabetes TB comorbidity” and “TB only group,” Chi-square test of significance was used, and unadjusted odds (UOR) ratio was reported. The variables having P < 0.20 in the bivariate analysis were included in the logistic regression analysis for adjustment. The output of regression analysis was reported as adjusted odds ratio with 95% confidence interval. All information obtained from the patients was kept confidential. The study was approved by the Institute Ethical Committee, and prior permission was taken from the State TB Cell, Chandigarh.
RESULTS
A total of 275 study participants were included giving the response rate of 92.9%. The mean age of the study participants was 34.2 years (standard deviation [SD] =15.5 years). There were 169 males (61.5%) and 106 females (38.5%). Sociodemographic details revealed that 26.5% were illiterate (73/275) and 34.3% were unemployed (94/275). Among the 275 participants, 157 (57.1%) were sputum smear-positive pulmonary TB patients, 20 were sputum smear-negative pulmonary TB (7.3%), 78 (28.4%) extrapulmonary TB, and 20 (7.3%) had MDR-TB.
The prevalence of DM among TB patients was 13.1% (36/275; (known diabetics 25/36 – 9.1% and new diabetics 11/36 – 4.0%). There were 1.8% (5/275) current smokers and 23.6% (65/275) former smokers. Regarding smokeless tobacco, 5.4% (15/275) were current smokeless tobacco and 7.6% (21/275) former smokeless tobacco users. Exposure to second smoke at home and workplace was present in 22.2% (61/275) and 17.8% (49/275) of study participants, respectively. In all, 44 of the 275 study participants had a family history of TB (16.0%).
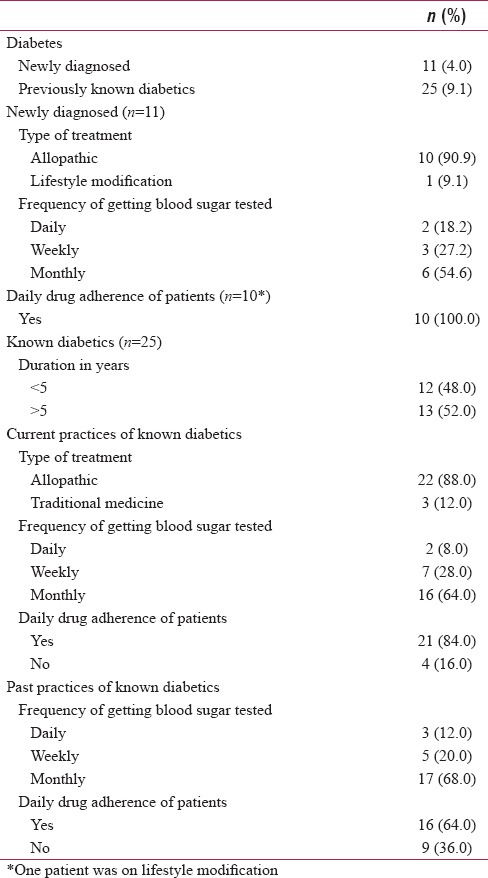
The mean age at the diagnosis of “known diabetics” was 44.6 years (SD = 9.4). Allopathic medicine was consumed by 88% (22/25); and the remaining took traditional medicines (3/25; 12%). Regularly antidiabetic daily medication was consumed by 84.0%. The proportion of “known diabetic” who got their blood sugar tested daily, weekly, monthly, was 8%, 28%, and 64%, respectively. In the past (i.e., before being diagnosed with TB), 16 out of 25 (64%) consumed antidiabetic medicines regularly. Previously, their blood sugar monitoring frequency on a daily, weekly, monthly, basis was 12%, 20%, and 68%, respectively. Out of 11 newly diagnosed cases of diabetes, 10 (90.9%) were on allopathic medication, and 1 (9.1%) was practising lifestyle modification. All new diabetics were taking drugs regularly on a daily basis (100.0%). The proportion of “new diabetic” who got their blood glucose tested daily, weekly, and monthly was 18.2%, 27.2%, and 54.6%, respectively [Table 1].
Table 1.
Self-reported medication, blood glucose monitoring practices, and control of diabetic tuberculosis patients
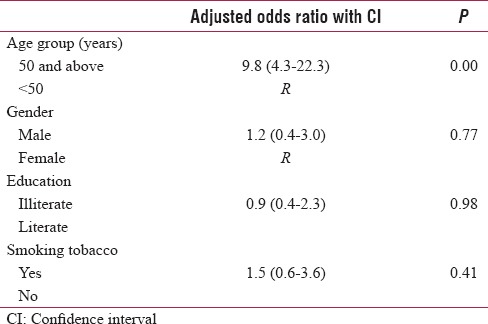
The bivariate analysis revealed that diabetes TB comorbidity patient's was significantly higher in tobacco smokers (UOR = 2.7 [1.3–5.6]) and those aged 50 years and above (UOR = 11.0 [5.1–23.9]) as compared to their counterparts. Smokeless tobacco use (UOR = 0.5 [0.2–1.9]), exposure to second-hand smoke (UOR = 0.9 [0.4–1.9]), and family history of TB (UOR = 0.8 [0.3–2.2]) had insignificant relation to diabetes TB comorbidity [Table 2]. In the logistic regression analysis, age 50 years and above emerged as a significant predictor for diabetes TB comorbidity (9.8 [4.3–22.3]). The Nagelkerke pseudo-R2 value for the regression model was 25.1% [Table 3].
Table 2.
Comparison of variables between “diabetes tuberculosis comorbidity” and “tuberculosis only group”
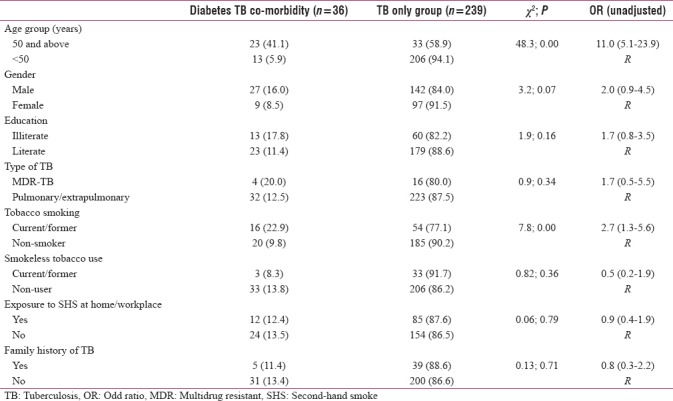
Table 3.
Logistic regression analysis of various factors associated with diabetes tuberculosis comorbidity
DISCUSSION
In the present study, the prevalence of DM in TB was 13.1%. This finding is comparable with the result of studies carried out in India (15.3%) and abroad (USA [11.4%]; Indonesia [14.8%]).[11,12,13] However, some studies conducted in India have reported comparatively higher prevalence ranging from 29% to 50%.[14,15] A low prevalence of diabetes in TB was observed in studies from China (6.3%) and Spain (5.9%).[16,17] A probable explanation for this variation in prevalence is the different study area and its population characteristics.
In the current study, the self-reported daily medication adherence of known diabetics was 84%. Before the diagnosis of TB, it was 64%. A low antidiabetic drug adherence can impact the TB treatment outcome. In a prospective study conducted by Siddiqui et al., it was reported that DM patients have poor treatment outcome.[18] Similarly, Viswanathan and Gawde reported that diabetes increases the risk of poor treatment outcomes among pulmonary TB patients.[19] In the current study, there were 25.5% of tobacco smokers. Similar to this finding, Mahishale et al. reported that 32% of TB patients were current and ex-smokers.[20] In another study conducted by Kolappan and Gopi, it was reported that 58% of the TB patients were smokers.[21] In our study, 13.1% of the study participants were current/former smokeless tobacco users. Studies conducted elsewhere have documented a high proportion of smokeless tobacco use among TB patients.[22,23]
In the present study, it was found that “TB diabetes comorbidity” was higher in those aged 50 years and above. Similar finding was reported in a study conducted by Achanta et al. in South India wherein those aged >40 years, we have more prevalence of diabetes.[24] In another study conducted by Nair et al., it was inferred that age >50 years was independently associated with a higher prevalence of diabetes in TB patients.[25] In this study, it was found that gender, type of TB, and smokings were not associated with TB. Contrary to our finding, a study conducted by Nagar et al. from Central India reported that smoking tobacco is an important risk factor for diabetes TB comorbidity.[26] A study done by Damtew et al. in Ethiopia reported that diabetes TB comorbidity was more among male than females.[27]
They are few limitations of the current study. The first is the self-reported assessment of DM as it could have resulted in disease underestimation. The diabetes status was, however, confirmed by prescription records and TB treatment cards. Secondly, a self-reported assessment of tobacco use and diabetes treatment practices/daily drug adherence was done. Thirdly, only those TB patients who were coming to TBHV of DMC for taking DOTS were enrolled in the study. Future studies can include patients enrolled with community DOTS provider as well. Finally, this study utilized a cross-sectional study design due to which causal relationship between diabetes TB comorbidity and associated factors cannot be established.
CONCLUSION
In the current study, every seventh TB patient was a diabetic and around one-fifth were current and former tobacco smokers. Increasing age is an important risk factor for TB diabetes comorbidity. It is recommended that diabetes screening in TB patients should be strengthened. Healthcare professionals should ensure optimal care for diabetic TB patients by conducting regular glycemic monitoring and improving medication adherence.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.TB India Report. [Last accessed on 2018 May 20]. Available from: https://www.tbcindia.gov.in/showfile. php?lid=3180 .
- 2.TB Comorbidities and Risk Factors. [Last accessed on 2018 May 20]. Available from: http://www.who.int/tb/ areas-of-work/treatment/risk-factors/en/
- 3.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Behera D. TB and diabetes – The dual epidemic: Is it a matter of concern? Indian J Tuberc. 2011;58:143–7. [PubMed] [Google Scholar]
- 5.Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lönnroth K, et al. The impact of diabetes on tuberculosis treatment outcomes: A systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamashiro S, Kawakami K, Uezu K, Kinjo T, Miyagi K, Nakamura K, et al. Lower expression of th1-related cytokines and inducible nitric oxide synthase in mice with streptozotocin-induced diabetes mellitus infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2005;139:57–64. doi: 10.1111/j.1365-2249.2005.02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Workneh MH, Bjune GA, Yimer SA. Prevalence and associated factors of diabetes mellitus among tuberculosis patients in South-Eastern Amhara region, Ethiopia: A cross sectional study. PLoS One. 2016;11:e0147621. doi: 10.1371/journal.pone.0147621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogbera AO, Kapur A, Abdur-Razzaq H, Harries AD, Ramaiya K, Adeleye O, et al. Clinical profile of diabetes mellitus in tuberculosis. BMJ Open Diabetes Res Care. 2015;3:e000112. doi: 10.1136/bmjdrc-2015-000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassmiller KM. The association between smoking and tuberculosis. Salud Publica Mex. 2006;48(Suppl 1):S201–16. doi: 10.1590/s0036-36342006000700024. [DOI] [PubMed] [Google Scholar]
- 10.National Framework for Joint TB-Diabetes Collaborative Activities. [Last accessed on 2018 Oct 03]. Available from: https://www.tbcindia.gov.in/WriteReadData/ National%20framework%20for%20joint%20TB%20diabetes%20 23%20Aug%202017.pdf .
- 11.Mansuri S, Chaudhari A, Singh S, Malek R, Viradiya R. Prevalence of diabetes among tuberculosis patients at urban health centre, Ahmedabad. Int J Sci Stud. 2015;3:115–8. [Google Scholar]
- 12.Magee MJ, Foote M, Maggio DM, Howards PP, Narayan KM, Blumberg HM, et al. Diabetes mellitus and risk of all-cause mortality among patients with tuberculosis in the state of Georgia, 2009-2012. Ann Epidemiol. 2014;24:369–75. doi: 10.1016/j.annepidem.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alisjahbana B, Sahiratmadja E, Nelwan EJ, Purwa AM, Ahmad Y, Ottenhoff TH, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis. 2007;45:428–35. doi: 10.1086/519841. [DOI] [PubMed] [Google Scholar]
- 14.Raghuraman S, Vasudevan KP, Govindarajan S, Chinnakali P, Panigrahi KC. Prevalence of diabetes mellitus among tuberculosis patients in urban Puducherry. N Am J Med Sci. 2014;6:30–4. doi: 10.4103/1947-2714.125863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balakrishnan S, Vijayan S, Nair S, Subramoniapillai J, Mrithyunjayan S, Wilson N, et al. High diabetes prevalence among tuberculosis cases in Kerala, India. PLoS One. 2012;7:e46502. doi: 10.1371/journal.pone.0046502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q, Ma A, Han X, Zhao S, Cai J, Ma Y, et al. Prevalence of type 2 diabetes among newly detected pulmonary tuberculosis patients in China: A community based cohort study. PLoS One. 2013;8:e82660. doi: 10.1371/journal.pone.0082660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno-Martínez A, Casals M, Orcau À, Gorrindo P, Masdeu E, Caylà JA, et al. Factors associated with diabetes mellitus among adults with tuberculosis in a large European city, 2000-2013. Int J Tuberc Lung Dis. 2015;19:1507–12. doi: 10.5588/ijtld.15.0102. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui AN, Khayyam KU, Sharma M. Effect of diabetes mellitus on tuberculosis treatment outcome and adverse reactions in patients receiving directly observed treatment strategy in India: A prospective study. Biomed Res Int 2016. 2016:7273935. doi: 10.1155/2016/7273935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viswanathan AA, Gawde NC. Effect of type II diabetes mellitus on treatment outcomes of tuberculosis. Lung India. 2014;31:244–8. doi: 10.4103/0970-2113.135764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahishale V, Patil B, Lolly M, Eti A, Khan S. Prevalence of smoking and its impact on treatment outcomes in newly diagnosed pulmonary tuberculosis patients: A hospital-based prospective study. Chonnam Med J. 2015;51:86–90. doi: 10.4068/cmj.2015.51.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolappan C, Gopi PG. Tobacco smoking and pulmonary tuberculosis. Thorax. 2002;57:964–6. doi: 10.1136/thorax.57.11.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mia MN, Hanifi SM, Rahman MS, Sultana A, Hoque S, Bhuiya A, et al. Prevalence, pattern and sociodemographic differentials in smokeless tobacco consumption in Bangladesh: Evidence from a population-based cross-sectional study in Chakaria. BMJ Open. 2017;7:e012765. doi: 10.1136/bmjopen-2016-012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deepak KG, Daivadanam M, Pradeepkumar AS, Mini GK, Thankappan KR, Nichter M, et al. Smokeless tobacco use among patients with tuberculosis in Karnataka: The need for cessation services. Natl Med J India. 2012;25:142–5. [PubMed] [Google Scholar]
- 24.Achanta S, Tekumalla RR, Jaju J, Purad C, Chepuri R, Samyukta R, et al. Screening tuberculosis patients for diabetes in a tribal area in South India. Public Health Action. 2013;3:S43–7. doi: 10.5588/pha.13.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nair S, Kumari AK, Subramonianpillai J, Shabna DS, Kumar SM, Balakrishnan S, et al. High prevalence of undiagnosed diabetes among tuberculosis patients in peripheral health facilities in Kerala. Public Health Action. 2013;3:S38–42. doi: 10.5588/pha.13.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagar V, Gour D, Pal DK, Singh AR, Joshi A, Dave L, et al. A study on prevalence of diabetes and associated risk factors among diagnosed tuberculosis patients registered under revised national tuberculosis control programme in Bhopal district. J Family Med Prim Care. 2018;7:130–6. doi: 10.4103/jfmpc.jfmpc_289_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damtew E, Ali I, Meressa D. Prevalence of diabetes mellitus among active pulmonary tuberculosis patients at St. Peter specialized hospital, Addis Ababa, Ethiopia. World J Med Sci. 2014;11:389–96. [Google Scholar]


