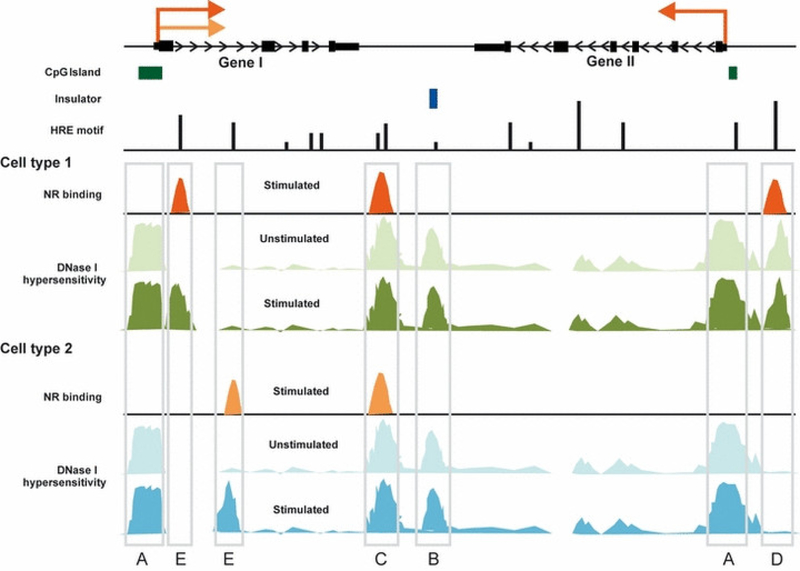
Fig. 1.
Tissue-specific chromatin architecture revealed in localization of DHSs. A schematic representation of DHSs before and after hormone stimulation in two cell types. The majority of hormone-responsive genes have a TSS that is embedded within a localized region of DNase I hypersensitivity. These promoter regions are generally hypersensitive across multiple cell types, and usually correlate with CpG islands (A). Common and preprogrammed DHSs present at distal regulatory elements often overlap with insulators (B). Hormone receptors recognize short DNA motifs (HREs), but only a small percentage of them are occupied by a receptor in a given cell type. NR binding occurs usually at distal enhancers and is highly correlated with the presence of accessible chromatin regions (C, D, E). Only a small fraction of enhancer-related DHSs are universally utilized in multiple cell lines and they usually represent hormone-independent chromatin structures (pre-programmed DHSs) (C). Most distal DHSs are tissue-specific and can be either hormone-independent (D) or appear only after hormone stimulation (inducible DHSs) (E). Thus the presence of a DHS and subsequent receptor/transcription factor binding results in a hormone-dependent and tissue-specific transcriptional regulation of a particular gene (gene II). A gene can be activated by the same hormone receptor in different tissues, although through different regulatory elements (gene I; elements C and E).