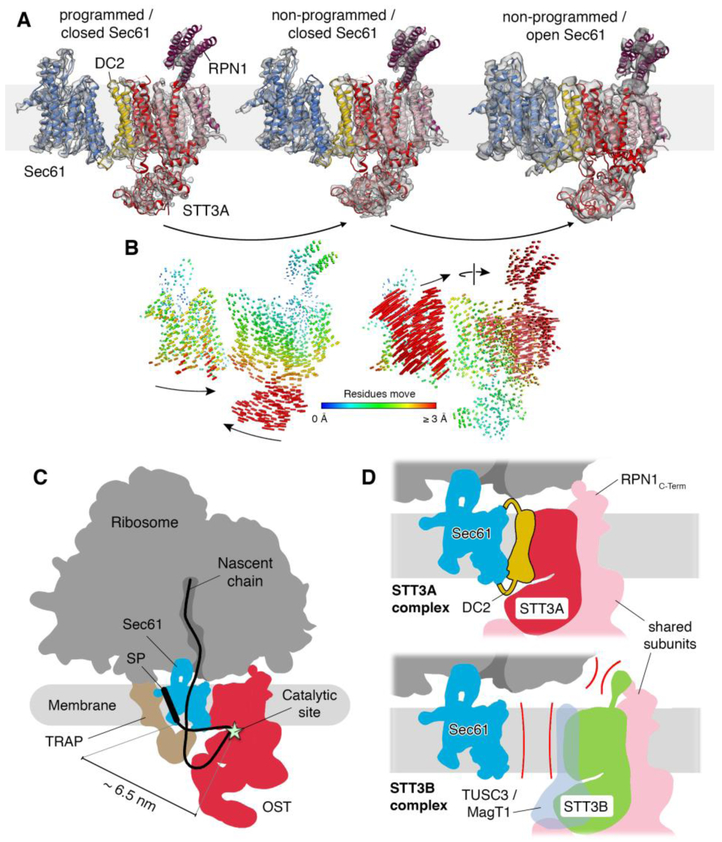
Fig. 3. Translocon dynamics and scheme for cotranslational N-glycosylation.
(A) Models for Sec61 and OST were fitted into the RTC densities with laterally closed (left: programmed, central: non-programmed) and opened Sec61 (right). (B) Trajectories of Cα atoms connecting the observed conformational states with color-coded length. (C) Schematic representation of the RTC with an interpolated example path for a nascent secretory protein. The STT3A catalytic site and a signal peptide (SP) or TM in the Sec61 lateral gate are separated by ~6.5 nm. (D) Molecular basis for STT3 paralog specificity in the RTC. The DC2 and RPN1 subunits tie the STT3A complex into the RTC (upper panel). The lack of DC2 and potential interference of the STT3B-specififc cytosolic domain with ribosome binding exclude STT3B complexes from the RTC.