Figure 2.
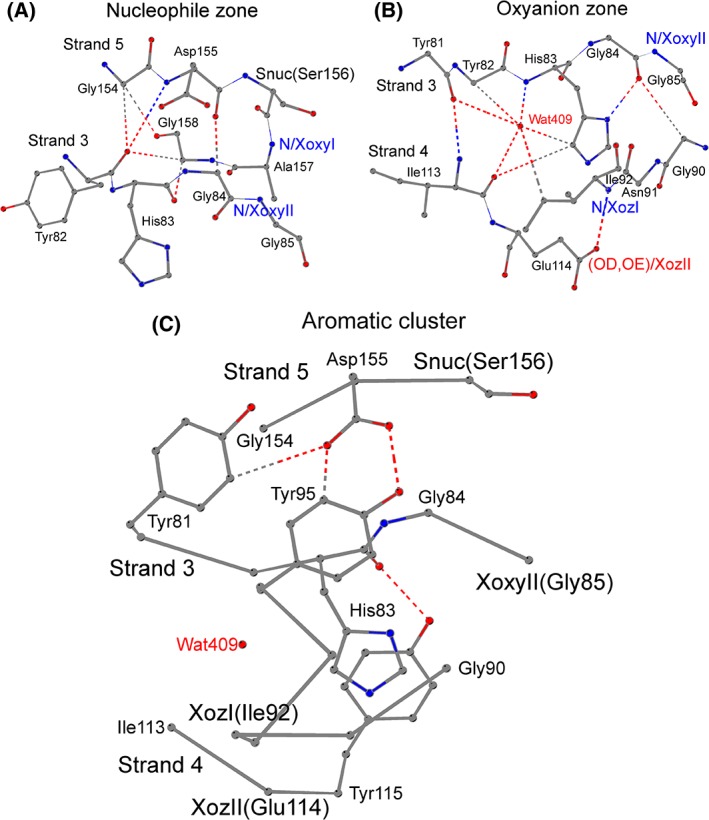
The nucleophile zone (A), the oxyanion zone (B), and the aromatic cluster (C) in the active site of ABH enzymes. (A) The nucleophile zone in the structure of the carboxylesterase EstFa_R from Ferroplasma acidiphilum (PDB ID: 3WJ2). The zone is shaped by the residues Asp155–Ser156–Ala157–Gly158 of the nucleophile elbow and His83–Gly84 at the start of loopβ3‐αA, and involves two, weak hydrogen bonds, O/Asp155–CA/Gly84 and CA/Gly158–O/His83. The residues Tyr82, Gly85, and Gly154 occupy semi‐conserved, peripheral positions and form four, supplementary hydrogen bonds to the nucleophile zone. “Snuc” designates the catalytic nucleophile residue, which is a serine in the majority of ABH enzymes; main‐chain nitrogen atoms at the oxyanion I (“N/XoxyI” – from Ala157 in EstFa_R) and Oxyanion II (“N/XoxyII” – from Gly85 in EstFa_R) positions form the oxyanion hole. (B) The oxyanion zone in the structure of the carboxylesterase EstFa_R from Ferroplasma acidiphilum (PDB ID: 3WJ2). The zone is formed by: the residues Tyr81–Tyr82–His83–Gly84 at the end of strand β3 and the start of loopβ3‐αA; the Gly90–Asn91–Ile92 at the end of loopβ3‐αA; and Ile113–Glu114 from strand β4. All amino acids except XoxyII, and all contacts except the structural β‐sheet hydrogen bond between Tyr81 and Ile113, belong to the oxyanion zone.“N/XoxyII” designates the main‐chain nitrogen atom of oxyanion II residue Gly85 in EstFa_R; and “Wat409” designates the conserved structural water molecule forming multiple hydrogen bonds in the oxyanion zone of the ABH enzymes. A hydrogen bond is formed between the main‐chain nitrogen atom of the residue at “oxyanion zone I” (“N/XozI”, Ile92 in EstFa_R) and the residue at “Oxyanion zone II” (“OD,OE/XozII”, side‐chain oxygen atom of Glu114 in EstFa_R). (C) Conserved residues at the aromatic cluster near the active site of the carboxylesterase EstFa_R from Ferroplasma acidiphilum (PDB ID: 3WJ2). The residues Tyr81, Tyr95, and Asp155 are located at positions where aromatic amino acids (as well as hydrophobic residues) are frequently found in all ABH enzymes; the side‐chain interactions of the residues at these positions form a “roof”‐like structural arrangement over the β‐sheet. Conservation of an aromatic residue is also observed before or after the residue at the position “oxyanion zone II” (“XozII”, Glu114 in EstFa_R); in EstFa_R, the side‐chain hydroxyl of Tyr115 interacts with the main‐chain oxygen of His83, and the two residues form an aromatic pair below the β‐sheet.“Snuc” and “XoxyII” designate the catalytic nucleophile and oxyanion II residue of the catalytic machinery, and “Wat409” and “XozI” designate the conserved structural water molecule and the residue at position “oxyanion zone I” of the Oxyanion zone.
