Figure 2.
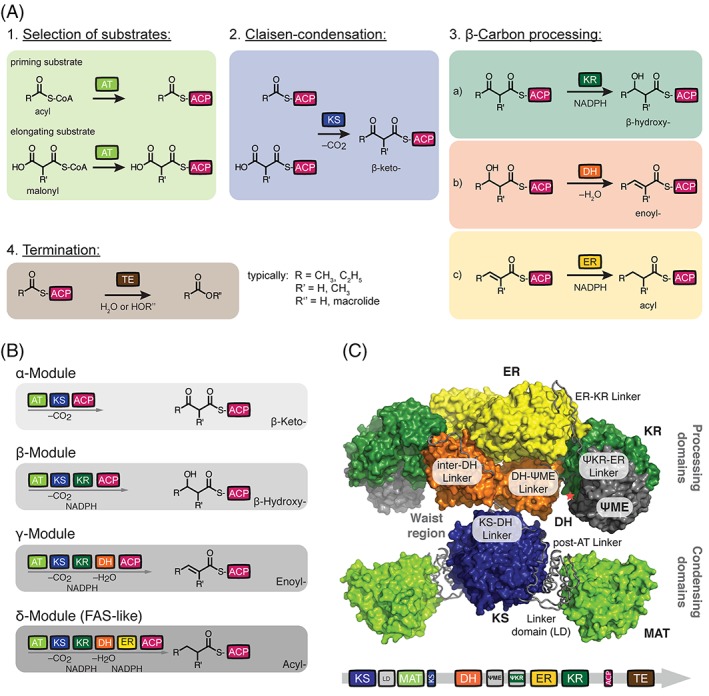
Architecture of animal FAS and PKS. (A) Commonly used domains of FASs and PKSs. Domain nomenclature: MAT, malonyl‐/acetyltransferase; AT, acyltransferase; KS, ketosynthase; KR, ketoreductase; DH, dehydratase; ER, enoylreductase; TE, thioesterase; ACP, acyl carrier protein; LD, linker domain; ψKR; truncated structural KR fold; ψME; truncated structural methyltransferase fold. (B) Domains are covalently connected in modules of FAS/PKS proteins. Essentially four module types appear in the FAS/PKS (cis‐AT PKS) family, and are termed α‐ (KS‐AT‐ACP), β‐ (KS‐AT‐KR‐ACP), γ‐ (KS‐AT‐DH‐KR‐ACP), and δ‐modules (KS‐AT‐DH‐ER‐KR‐ACP). The different modules elongate and modify the polyketide intermediate. In a first of putatively several elongation cycles, R and R’ refer to typically CH3 and C2H5 as well as H and CH3, respectively. Color code as introduced in (a). (C) X‐ray structure of animal FAS with domains and inter‐domain linkers in surface and tube representation, respectively (porcine FAS, PDB accession code 2vz9).12 Red asterisk indicates the C‐terminus of the KR domain, where the ACP and TE domains are flexibly tethered. TE and ACP could not be traced in electron density in the X‐ray structure. Color code as introduced in (a).
