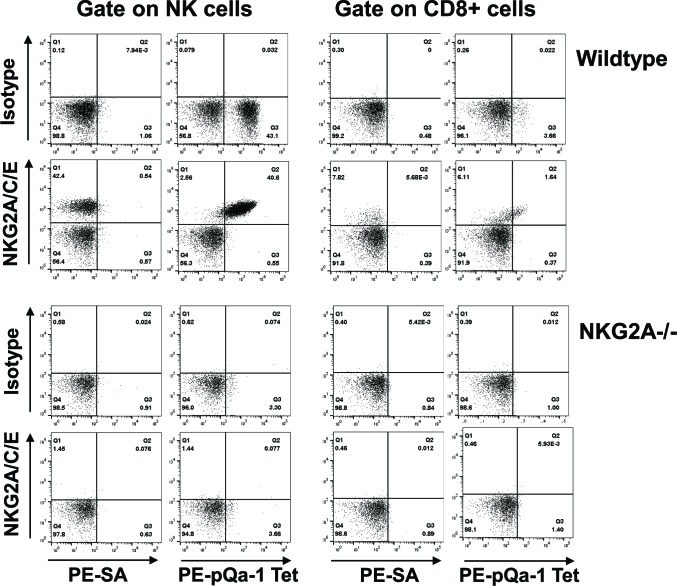
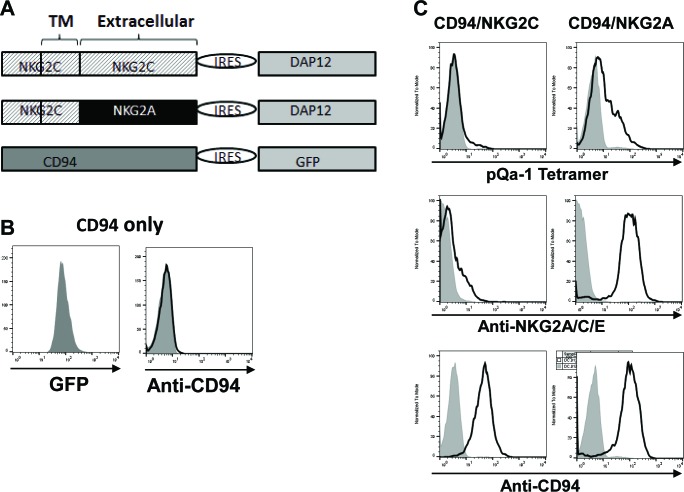
Figure 5. The Qdm-β2m-pQa-1 single chain trimer (SCT) specifically recognizes the CD94/NKG2A receptor.
(A) Spleen lymphocytes isolated from wildtype C57BL/6 or NKG2A-/- knockout mice were stained with PE-labeled pQa-1-dtSCT tetramer (PE-pQa-1 Tet), or PE-labeled streptavidin (PE-SA) as negative control, at room temperature for 1 hr followed by staining with a mixture of fluorochrome labeled antibodies containing either anti-NKG2A/C/E (20D5) or isotype control for 30 min. The stained cells were acquired by BD Canto II and data were analyzed with FlowJo software. Representative data of four (wildtype) and two (NKG2A-/-) independent experiments are shown. NK cells were defined as the live NK1.1+CD3-CD19- population, while CD8 T cells were gated on the live CD19- CD3+CD8+ population.
Figure 5—figure supplement 1. pQa-1 single chain trimer design and increased thermal stability.