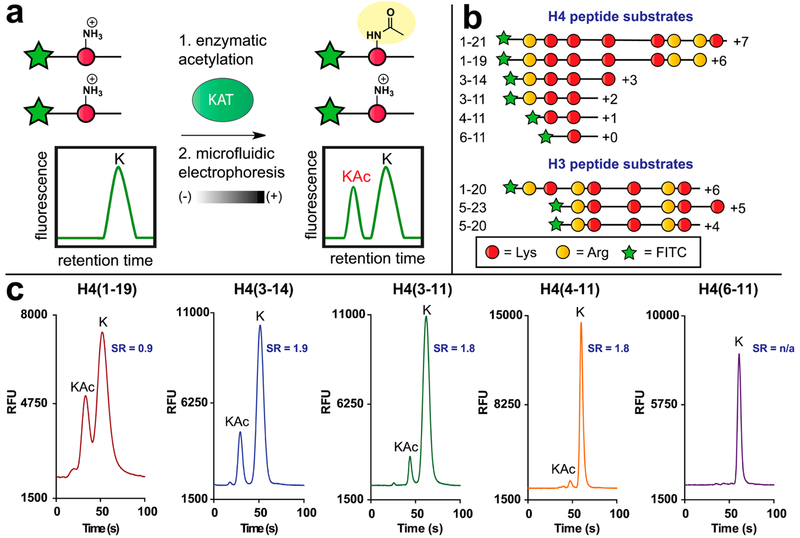
Figure 1.
Microfluidic mobility shift profiling of lysine acetyltransferase activity. (a) Assay principle. Fluorescent KAT substrate peptides are incubated with KAT and acetyl-CoA. Acetylation of substrate alters its charge-to-mass ratio, enabling electrophoretic separation and direct measurement of KAT enzyme activity. (b) Schematic and net charges of histone H4/H3 KAT substrate peptides used in this study. All peptides were modified with an N-terminal aminohexanoic acid-FITC to facilitate detection. Sequences of each fluorescent substrate are provided in Table S1. (c) Charge-dependent separation of acetylated (“KAc”) and nonacetylated (“K”) fluorescent histone H4 substrates. SR = separation resolution.