Abstract
Supporting cells (SCs) provide structure and maintain an environment that allows hair cells to receive and transmit signals in the auditory pathway. After insult to hair cells and ganglion cells, SCs respond by marking unsalvageable cells for death and maintain structural integrity. While the histopathology after cochlear implantation has been described regarding hair cells and neural structures, surviving SCs in the implanted ear has not. We present a patient whose posthumous examination of an implanted cochlea demonstrated SC survival. This finding has implications for SC function in maintaining electrical hearing and candidacy for future hair cell regeneration therapies.
Keywords: Cochlear implant, supporting cell, histopathology, spiral ligament, endolymphatic hydrops
INTRODUCTION
Significant attention has been given to cochlear hair cells which serve as the sensory receptors for sound detection. However, supporting cells (SCs) serve equally important roles in the development, function, and maintenance of the inner ear. Homeostasis and structural integrity of the organ of Corti are provided by SCs.1 As reviewed by Monzack and Cunningham, cochlear SCs are responsible for critical functions in the inner ear including the maintenance of both spiral ganglion neurons and hair cells.2 In response to ototoxic stress causing damage or death of hair cells and spiral ganglion neurons, SCs serve to limit the extent of injury and facilitate recovery.3 In the presence of damage to hair cells, avian auditory and vestibular SCs as well as some mammalian vestibular SCs have the capacity to transdifferentiate and replace hair cells.4 However, in the adult mammalian cochlea, mature auditory hair cells cannot regenerate after injury which results in permanent hearing loss.
Currently, cochlear implantation in cases of severe to profound sensorineural hearing loss (SNHL) circumvents this irreversible loss of hair cells by bypassing the damaged portions of the ear and directly stimulating the remaining spiral ganglion neurons and cochlear nerve fibers.5 Expansion of cochlear implant (CI) candidacy criteria have allowed patients with more residual low-frequency hearing to undergo cochlear implantation, which raised an important issue of preserving residual hair cells to optimize the outcomes of these patients. Furthermore, targeting cochlear SCs to either regenerate into hair cells or to promote survival of hair cells and spiral ganglion neurons in patients with CIs may assist in efforts to optimize CI outcomes. However, the viability of SCs after cochlear implantation has not been investigated previously. Here we present the human temporal bone histopathology from one such patient who underwent cochlear implantation in life and was found to have surviving SCs posthumously. This significant finding suggests that cochlear implantation may not preclude patients from future therapies that may target SCs for hair cell regeneration.
CASE REPORT
The patient was an 84-year-old male with progressive bilateral SNHL due to otosclerosis who underwent a CI with the Nucleus 22 channel electrode CI (Cochlear Americas, CO, USA) in the right ear. The patient exhibited a profound bilateral SNHL with absent speech discrimination bilaterally at implantation as seen in Figure 1. Progression of hearing loss is shown between initial audiogram 23 years prior and preoperative audiogram (Figure 1).
Figure 1.
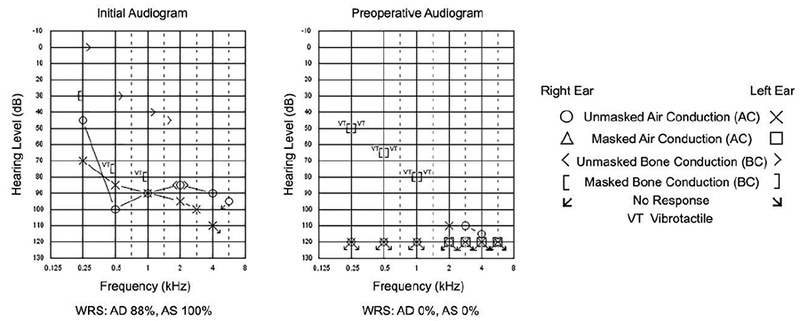
Initial and preoperative audiograms in patient with bilateral progressive sensorineural hearing loss due to otosclerosis. Initial audiogram 23 years prior shows a moderate mixed hearing loss at 250 Hz sloping to profound SNHL from 500 to 8000 Hz in the right ear and a severe mixed hearing loss at 250 Hz sloping to profound mixed hearing loss from 1000Hz and above in the left ear. Right air conduction pure tone average (PTA) was 92 dB. Air and bone conduction PTA were 83 dB and 50 dB, respectively in the left ear. Speech discrimination scores in the right and left ears was 88% and 100%, respectively. Preoperative audiogram demonstrates a profound bilateral sensorineural hearing loss (SNHL) with absent speech discrimination bilaterally. Legend for audiogram symbols is provided to the right of the audiograms.
Cochlear implant insertion was performed utilizing an expanded round window cochleostomy technique. Insertion was incomplete with seven to eight rings outside of the basal turn. The basal turn at the cochleostomy site and the facial recess were packed with soft tissue. There were no documented surgical complications. The patient passed away three years later of unknown causes.
Histologic Preparation
Research was conducted under UCLA IRB approved protocol # 10–001449-CR-0007. The temporal bones were removed postmortem with the implant device retained within the specimen and sectioned in the axial plane at a thickness of 20 micrometers. Detailed procedures of temporal bone collection, fixation, decalcification and celloidin embedding were described by Merchant.6 The methodology to mount celloidin-embedded sections, to perform celloidin removal and antigen retrieval steps, and to perform immunohistochemistry on human temporal bones has been described previously.7 Sections were incubated with primary antibodies for 48 h at room temperature and secondary antibodies for 2 h at room temperature. Sections were stained with mouse anti-Acetylated tubulin (1:250; Sigma), a known marker for adult cochlear SCs, and 4’,6-diamidino-2-phenylindole (DAPI) to stain nuclear structures. Confocal laser scanning microscopy imaging was performed on sections to obtain images. The CI electrode is removed prior to decalcification and sectioning leaving behind the fibrous tissue reaction surrounding the electrode.
Histopathologic Findings
In the implanted right temporal bone, the electrode passes from the middle ear through a widened round window area into the scala tympani where it is encased in fibrous tissue and new bone. The electrode migrates at the anterior curvature of the cochlea to occupy the spiral ligament region (Figure 2). In this region, the organ of Corti is absent and the spiral ligament (SL) is confluent with loose areolar tissue of the scala tympani (ST) (Figure 2A). The electrode erodes through the endosteal layer of the bone at the superior anterior portion of the basal turn (double arrowheads), with evidence of suppuration surrounded by new bone formation as denoted by the asterisk and arrowheads, respectively (Figure 2B). The electrode passes as far as the upper basal turn. Mild endolymphatic hydrops is seen throughout the cochlea which is more notable in the apical and middle turns compared to the basal turn (Figure 3A) with comparison to a patient with normal hearing and normal caliber of the scala media (Figure 3B). The organ of Corti is represented by a mound of confluent cells in the middle and apical portions of the cochlea. The spiral ligament is damaged around the electrode path but is otherwise normal. The right ear is also notable for oval window otosclerosis penetrating the endosteal bone of the cochlea. In the unoperated left temporal bone, hair cells and SCs are largely preserved with minimal loss in the basal turn (data not shown). Otosclerotic lesions are seen at the stapes footplate, oval window, round window partially entering the scala tympani, and internal auditory canal. Spiral ganglion cell counts were 13,693 in the implanted right ear and 19,404 in left ear.
Figure 2.
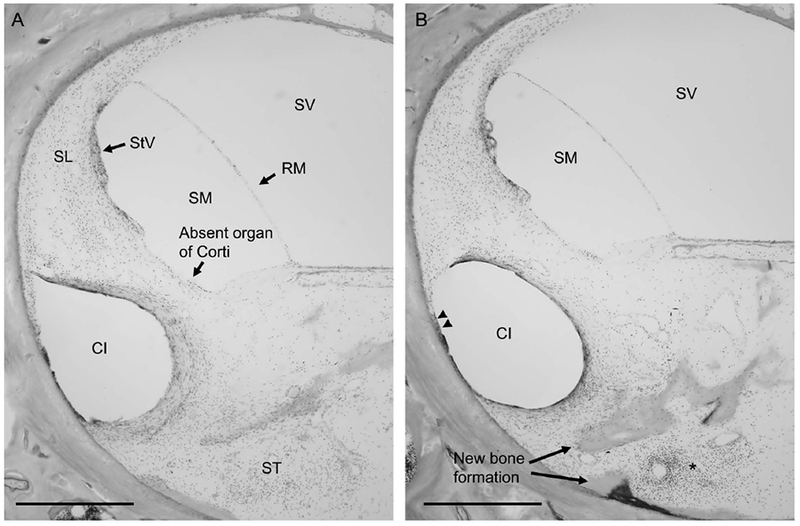
Description of cochlear implant (CI) insertion trauma to spiral ligament in basal turn of the cochlea.Figure 2A, The impression of the CI electrode migrates at the anterior curvature of the cochlea to occupy the spiral ligament, which is confluent with the loose areolar tissue of the scala tympani (ST). Note the absent organ of Corti in this region of the basal turn of the cochlea. Figure 2B, CI is seen to erode through the endosteal layer of the bone at the superior anterior portion of the basal turn (double arrowheads), with evidence of suppuration (asterisk) surrounded by new bone formation (arrowheads).
SL = spiral ligament, RM = Reissner’s membrane, StV = stria vascularis, SM = scala media, ST = scala tympani, SV = scala vestibuli, CI = cochlear implant electrode impression, double arrowheads = erosion of CI electrode through endosteal layer of the bone into marrow spaces, arrows = new bone formation. Scale bar, 500 μm.
Figure 3.
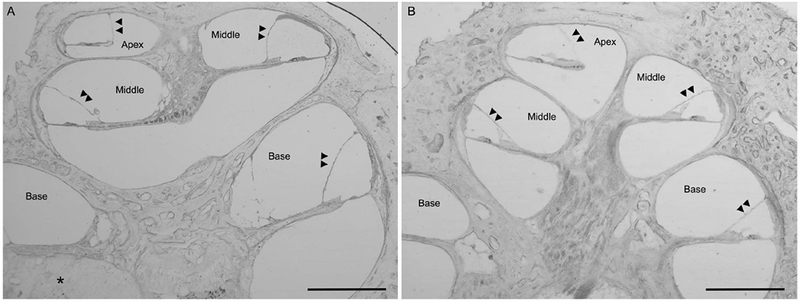
Presence of endolymphatic hydrops in the patient with a multichannel cochlear implant (3A, LEFT) with comparison to a normal hearing patient (3B, RIGHT). Mid-modiolar cross-sections, low-magnification light microscopy images, magnification 2.5x; Scale bar, 1mm. Double arrowheads point the location of Reissner’s membrane. Figure 3A, Right ear of our patient with a multichannel cochlear implant. Note the presence of endolymphatic hydrops (bulging of Reissner’s membrane with expansion of the scala media with the double arrowheads pointing to Reissner’s membrane) in the apical and middle turns with less noticeable but present endolymphatic hydrops in the basal turn. Area of fibrosis around the cochlear implant electrode denoted by the asterisk. Figure 3B, Comparison image from a patient with normal hearing and normal caliber of the scala media.
Fluorescence confocal laser scanning microscopy was performed on sections of organ of Corti in the apex, middle and base of the cochlea (Figure 4). Organ of Corti regions are boxed in the apex, middle and base, respectively in the low-magnification light microscopy image (Figure 4A). Confocal laser scanning microscopy images of the organ of Corti in the apex, middle and base of the cochlea are shown stained with DAPI for nuclear staining (in white) and acetylated tubulin (in red), a known adult cochlear SC marker (Figure 4B). Hair cells, both inner and outer hair cells, are absent. Arrows and brackets mark the approximate location of inner and outer hair cells, respectively. Grayscale images of acetylated tubulin staining are shown in Figure 4C. Pillar, Deiter, Hensen, and Claudius SCs are demonstrated in the apex (Figure 4C), middle (Figure 4D), and basal (Figure 4F) segments.
Figure 4.
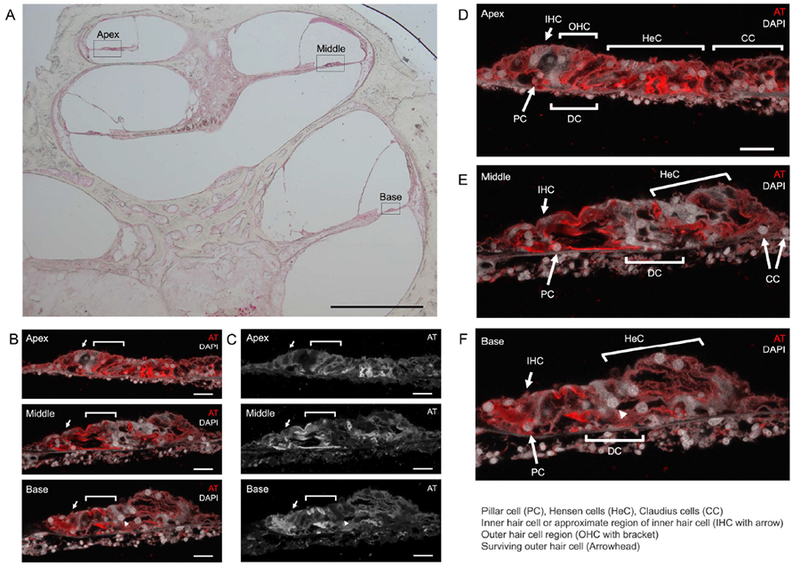
Survival of adult cochlear supporting cells after cochlear implant. Figure 4A, Mid-modiolar cross-section light microscopy image, black boxes identify the regions corresponding to confocal laser scanning microscopy images 4B-4F (Magnification 2.5x; Scale bar, 1 mm). Figure 4B (color) and 4C (grayscale), Confocal laser microscopy images of organ of Corti in the apex, middle and base (Magnification 40x; Scale bar, 20 μm). Acetylated tubulin (AT) and 4’6’-diamidino-2-phenylindole (DAPI) label adult supporting cells and nuclei, respectively. Notice that hair cells are largely absent. A surviving hair outer hair cell (white arrowhead) is demonstrated in the basal turn of the organ of Corti (Notice that it is not labeled with acetylated tubulin in the grayscale image). Figure 4D, 4E, 4F respectively represent larger color images of apex, middle, and base of the cochlea seen in Figure 4B. IHC = inner hair cell or approximate region of inner hair cell, OHC = outer hair cell region, PC = Pillar cell, DC = Deiter cells, HeC = Hensen cells, CC = Claudius cells. White arrows and brackets mark the approximate regions for the inner hair cells and outer hair cells, respectively.
DISCUSSION
The histologic changes associated with cochlear implantation have been previously described.8–10 In our patient, these findings include fibrous tissue deposition, new bone formation, and endolymphatic hydrops. Although we did not see fracture of the osseous spiral lamina, modiolus, or tear of the stria vascularis, erosion of the endosteal layer of the basal turn due to electrode insertion may be equivalent to severe or Eshraghi grade 4 trauma.11 This is consistent with the grade of trauma often seen with an expanded round window technique.12 By immunohistochemistry, we demonstrate the presence of SCs in the basal, middle, and apical portions of the cochlea despite high grade electrode insertion trauma.
Expansion of CI indications have resulted in an increasing number of patients with residual hearing receiving CIs.13 In this setting, preservation of residual hearing has become a clinical priority and mechanisms by which this goal can be accomplished have become an area of intense focus. This is particularly challenging because direct tissue trauma from CI electrode insertion can trigger the caspase pathway and programmed cell death in hair cells resulting in additional loss of residual hearing.14 Cochlear SCs have been shown to support the survival of both spiral ganglion neurons and cochlear hair cells.15 Thus, the finding of surviving SCs after CI has several clinically significant implications: 1) the presence of a CI may not preclude patients from future attempts at regenerating hair cells from SCs, and 2) SCs can continue their normal function of maintaining cochlear structure, promoting hair cell fate and supporting spiral ganglion cell survival. Future interventions that can augment these inherent abilities of SCs to maintain spiral ganglion neurons and cochlear hair cells may serve to optimize CI outcomes. We briefly review these SC functions.
Hearing loss resulting from permanent hair cell damage or death can occur by many mechanisms, including aging, acoustic trauma, and ototoxic medications. In the setting of aminoglycoside or loop diuretic drugs, SCs in guinea pig cochlea have been demonstrated to induce heat shock proteins to attempt to protect hair cells from damage.16 However, if the damage is extensive and death is inevitable, SCs can activate apoptotic pathways in damaged hair cells, phagocytose dying hair cells and expand to maintain reticular lamina integrity.17–19
Currently, the complete cascade of transcription and epigenetic factors necessary to drive transdifferentiation of adult cochlear SCs into hair cells and restore hearing remain undefined. A proposed regenerative strategy may include activation of the Wnt pathway, which stimulates cell proliferation in neonatal cochlear SCs, followed by Notch inhibition to direct hair cell differentiation.20 Although further work is needed in this realm, surviving SCs in the setting of CI represent a potential future substrate for hearing restoration.
Limitations of our study include the partial electrode insertion and single patient study. The degree of damage and supporting cell survival may differ in a patient with a full insertion. However, in our patient, we demonstrated supporting cell survival despite high grade cochlear trauma in the basal region in which the electrode passed. A larger histologic study including full insertions with various electrode types would be needed to corroborate our findings.
CONCLUSIONS
Supporting cells of the inner ear serve many critical purposes in normally functioning ears and in those with hair cell and spiral ganglion loss. The finding of intact, surviving SCs in an implanted cochlea suggests that CI users may not be excluded from future therapies directed towards hair cell regeneration from SCs. Similarly, future interventions which can enhance the role of SCs in maintaining existing hair cells and spiral ganglion neurons may assist in optimizing CI outcomes.
Acknowledgments
FUNDING: This work was supported by an NIDCD Intramural Research Program fund 1ZIADC000088–02 (to M.H.). I.L., F.H.L. and A.I. were supported by NIDCD extramural research fund 1U24DC015910–01 (to A.I.).
Footnotes
CONFLICTS OF INTEREST: None
REFERENCES
- 1.Oesterle EC, Campbell S. Supporting cell characteristics in long-deafened aged mouse ears. J Assoc Res Otolaryngol 2009;10(4):525–544. doi: 10.1007/s10162-009-0183-x [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monzack EL, Cunningham LL. Lead roles for supporting actors: Critical functions of inner ear supporting cells. Hear Res 2013;303:20–29. doi: 10.1016/j.heares.2013.01.008 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francis SP, Cunningham LL. Non-autonomous cellular responses to ototoxic drug-induced stress and death. Front Cell Neurosci 2017;11:252. doi: 10.3389/fncel.2017.00252. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warchol ME. Sensory regeneration in the vertebrate inner ear: Differences at the levels of cells and species. Hear Res 2011;273(1–2):72–79. doi: 10.1016/j.heares.2010.05.004. [doi] [DOI] [PubMed] [Google Scholar]
- 5.Lin V, Golub JS, Nguyen TB, Hume CR, Oesterle EC, Stone JS. Inhibition of notch activity promotes nonmitotic regeneration of hair cells in the adult mouse utricles. J Neurosci 2011;31(43):15329–15339. doi: 10.1523/JNEUROSCI.2057-11.2011. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merchant S 1: Methods of removal, preparation, and study In: Schuknecht pathology of the ear. Shelton, CT: PMPH-USA; 2010:3–51. [Google Scholar]
- 7.Lopez IA, Ishiyama G, Hosokawa S, et al. Immunohistochemical techniques for the human inner ear. Histochem Cell Biol 2016;146(4):367–387. doi: 10.1007/s00418-016-1471-2. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fayad JN, Linthicum FH Jr. Multichannel cochlear implants: Relation of histopathology to performance. Laryngoscope. 2006;116(8):1310–1320. doi: 10.1097/01.mlg.0000227176.09500.28 [doi]. [DOI] [PubMed] [Google Scholar]
- 9.Li PM, Somdas MA, Eddington DK, Nadol JB Jr. Analysis of intracochlear new bone and fibrous tissue formation in human subjects with cochlear implants. Ann Otol Rhinol Laryngol 2007;116(10):731–738. doi: 10.1177/000348940711601004. [doi] [DOI] [PubMed] [Google Scholar]
- 10.Quesnel AM, Nakajima HH, Rosowski JJ, Hansen MR, Gantz BJ, Nadol JB Jr. Delayed loss of hearing after hearing preservation cochlear implantation: Human temporal bone pathology and implications for etiology. Hear Res 2016;333:225–234. doi: S0378-5955(15)00185-9 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eshraghi AA, Yang NW, Balkany TJ. Comparative study of cochlear damage with three perimodiolar electrode designs. Laryngoscope. 2003;113(3):415–419. Accessed Jun 17, 2018. doi: 10.1097/00005537-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Richard C, Fayad JN, Doherty J, Linthicum FH. Round window versus cochleostomy technique in cochlear implantation: Histologic findings. Otol Neurotol. 2012;33(7):1181–1187. Accessed Jun 17, 2018. doi: 10.1097/MAO.0b013e318263d56d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gantz BJ, Dunn CC, Oleson J, Hansen MR. Acoustic plus electric speech processing: Long-term results. Laryngoscope. 2017. doi: 10.1002/lary.26669 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eshraghi AA, Lang DM, Roell J, et al. Mechanisms of programmed cell death signaling in hair cells and support cells post-electrode insertion trauma. Acta Otolaryngol. 2015;135(4):328–334. Accessed Jun 17, 2018. doi: 10.3109/00016489.2015.1012276. [DOI] [PubMed] [Google Scholar]
- 15.Takada Y, Takada T, Lee MY, et al. Ototoxicity-induced loss of hearing and inner hair cells is attenuated by HSP70 gene transfer. Mol Ther Methods Clin Dev 2015;2:15019. doi: 10.1038/mtm.2015.19 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anttonen T, Herranen A, Virkkala J, et al. C-jun N-terminal phosphorylation: Biomarker for cellular stress rather than cell death in the injured cochlea. eNeuro. 2016;3(2):Apr. doi: 10.1523/ENEURO.0047-16.2016 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anttonen T, Belevich I, Kirjavainen A, et al. How to bury the dead: Elimination of apoptotic hair cells from the hearing organ of the mouse. J Assoc Res Otolaryngol 2014;15(6):975–992. doi: 10.1007/s10162-014-0480-x [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forge A Outer hair cell loss and supporting cell expansion following chronic gentamicin treatment. Hear Res 1985;19(2):171–182. [DOI] [PubMed] [Google Scholar]
- 19.Teufert KB, Linthicum FH Jr, Connell SS. The effect of organ of corti loss on ganglion cell survival in humans. Otol Neurotol. 2006;27(8):1146–1151. doi: 10.1097/01.mao.0000232006.16363.44. [doi] [DOI] [PubMed] [Google Scholar]
- 20.Atkinson PJ, Huarcaya Najarro E, Sayyid ZN, Cheng AG. Sensory hair cell development and regeneration: Similarities and differences. Development. 2015;142(9):1561–1571. Accessed Jun 17, 2018. doi: 10.1242/dev.114926. [DOI] [PMC free article] [PubMed] [Google Scholar]


