Abstract
The development of acute kidney injury (AKI) is a complex process involving tubular, inflammatory, and vascular components, but less is known about the role of the interstitial microenvironment. We have previously shown that the extracellular matrix glycoprotein tenascin-C (TNC) is induced in fibrotic kidneys. In mouse models of AKI induced by ischemia-reperfusion injury (IRI) or cisplatin, TNC was induced de novo in the injured sites and localized to the renal interstitium. The circulating level of TNC protein was also elevated in AKI patients after cardiac surgery. Knockdown of TNC by shRNA in vivo aggravated AKI after ischemic or toxic injury. This effect was associated with reduced renal β-catenin expression, suggesting an impact on Wnt signaling. In vitro, TNC protected tubular epithelial cells against apoptosis and augmented Wnt1-mediated β-catenin activation. Co-immunoprecipitation revealed that TNC physically interacts with Wnt ligands. Furthermore, a TNC-enriched kidney tissue scaffold prepared from IRI mice was able to recruit and concentrate Wnt ligands from the surrounding milieu ex vivo. The ability to recruit Wnt ligands in this ex vivo model diminished after TNC depletion. These studies indicate that TNC is specifically induced at sites of injury and recruits Wnt ligands, thereby creating a favorable microenvironment for tubular repair and regeneration after AKI.
Keywords: Tenascin-C, Wnt/β-catenin, AKI, apoptosis, injury repair, tissue scaffold
INTRODUCTION
Acute kidney injury (AKI) is a devastating clinical condition characterized by an abrupt loss of kidney function after injury. AKI is associated with high morbidity and mortality, and it accounts for approximately 2 million deaths each year worldwide.1–3 AKI affects 7~18% of the hospital inpatients and 20~200 individuals per million population in the community.4, 5 Despite a certain degree of renal repair and recovery, severe or repeated AKI often transition into chronic kidney disease (CKD) or end-stage renal failure, in which life-sustaining renal replacement therapies are needed.6, 7
The pathophysiology of AKI is complex, which involves many types of residential and infiltrated cells in the kidneys.8, 9 Upon ischemic or nephrotoxic insults, tubular cells are damaged and often undergo a series of intracellular changes involving oxidative stress,10 mitochondrial dysfunction,11 apoptosis12 and necrosis.13 These tubular injuries are accompanied by renal vascular impairment and the infiltration of inflammatory cells.14–17 While the involvement of tubular, inflammatory and vascular components in the pathogenesis of AKI is extensively studied and well established, little is known about the impact of renal interstitial microenvironment in regulating kidney injury and repair after AKI.
Tenascin-C (TNC) is a large hexametric extracellular matrix (ECM) glycoprotein, which plays an imperative role in organizing a special tissue microenvironment.18, 19 Structurally, TNC consists of several unique domains, including an N-terminal oligomerization domain, tandem epidermal growth factor-like repeats, fibronectin type III-like repeats and a fibrinogen homology domain.18 TNC is highly expressed during embryonic development, but its expression is generally silenced in adults. De novo induction of TNC is observed in many organs under a variety of pathologic conditions, such as tissue injury and repair, matrix remodeling and fibrosis, inflammation and tumorigenesis.20–22 As a matricellular protein, TNC is not obligatory for maintaining ECM structural integrity, but it elicits critical biologic actions via binding to other growth factors and ECM proteins, as well as cell membrane receptors such as integrins or toll-like receptors.23–27 Growing evidence suggests that TNC is essential for orchestrating the formation of stem cell or progenitor cell niche.19, 24, 28 We recently showed that TNC is markedly induced in the fibrotic kidneys, and it functions as an organizer of the fibrogenic niche supporting fibroblast proliferation.20 However, whether TNC plays any role in constructing a microenvironment that regulates kidney injury and repair in the setting of AKI remains unknown.
In this study, we investigated TNC expression and function in both ischemic and toxic models of AKI. Our results indicate that TNC is renal protective in AKI by potentiating Wnt/β-catenin signaling. By using decellularized kidney tissue scaffold (KTS), we demonstrate that TNC is able to recruit and concentrate Wnt ligands, thereby creating favorable microenvironment for tubular repair and regeneration. Our studies for the first time illustrate the importance of the TNC-rich microenvironment in controlling kidney injury and repair after AKI.
RESULTS
De novo induction of renal TNC in various models of AKI
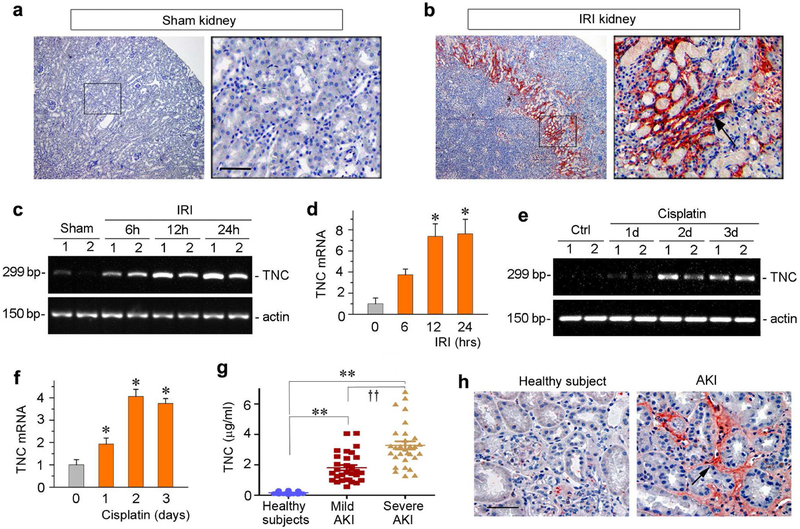
To study the regulation of TNC in AKI, we first used mouse model of ischemia/reperfusion injury (IRI). As shown in Figure 1, a and b, TNC protein was absent in sham-operated kidney, but its expression was dramatically upregulated at 30 hours after IRI. Notably, TNC induction was largely limited to the junctional region of renal cortex and medulla, an area well known to be main injurious site in this model. The cellular localization of TNC protein appeared in the interstitial compartment of renal parenchyma (Figure 1b, arrow). To assess the dynamics of TNC induction in IRI, we examined renal TNC mRNA expression by RT-PCR at different time points. As shown in Figure 1, c and d, renal TNC mRNA was induced as early as 6 hours, and continued to increase at 12 and 24 hours after IRI. These results suggest that TNC induction is an early and specific event that occurs predominantly at the sites of injury after IRI.
Figure 1. Expression of TNC is induced in animal models of AKI and in humans.
(a, b) Representative micrographs show TNC protein expression in sham and ischemic kidney at 30 hours after IRI. Kidney sections were stained immunohistochemically with specific antibody against TNC. Boxed area was enlarged. Arrow indicates positive staining. Scale bar, 50 μm. (c, d) RT-PCR results show the relative mRNA abundances of TNC at different time points after IRI. Representative RT-PCR results (c) and quantitative data (fold induction over the controls) (d) are presented. *P < 0.05 versus controls (n = 5 to 6). (e, f) RTPCR results show the relative mRNA abundances of TNC at different time points after cisplatin. Representative RT-PCR results (e) and quantitative data (fold induction over the controls) (f) are presented. *P < 0.05 versus controls (n = 5 to 6). (g) The circulating levels of TNC are elevated in human AKI. TNC protein was detected by a specific ELISA in the plasma of normal healthy subjects and patients with mildand severe AKI after cardiac surgery, respectively. **P< 0.01 versus healthy subjects; ††P< 0.01 versusmild AKI. (h) Representative micrographs show the abundance and localization of TNC proteins in healthy control and AKI patients as indicated. Scale bar, 50 μm.
To examine whether TNC is also induced in other forms of AKI, we investigated its expression in cisplatin nephropathy. As shown in Figure 1, e and f, renal TNC mRNA was induced at different times after cisplatin injection, suggesting that TNC induction is a common feature that occurs in toxic AKI as well.
Induction of TNC in human AKI
To assess the clinical relevance of TNC upregulation to human AKI, we measured human plasma levels of TNC in normal healthy subjects and a cohort of 60 AKI patients after cardiac surgery using a specific ELISA. The demographic and clinical features of these patients were presented in Supplementary Table 1. As shown in Figure 1g, TNC protein in the circulation was markedly elevated in AKI patients at 48 hours after cardiac surgery. Notably, the levels of plasma TNC were closely correlated with the severity of kidney injury(Figure 1g). In the patients who developed severe AKI, TNC levels were increased dramatically, comparedto that in healthy subjects or in patients with mild AKI. (Figure 1g). We also analyzed TNC expression in human kidney biopsies from patients with AKI. As shown in Figure 1h, TNC was also upregulated in the tubulointerstitial space of kidneys in AKI patients. Therefore, induction of TNC is a generalized response of the kidneys to acute injury in mice and humans.
Knockdown of TNC aggravates kidney injury after AKI
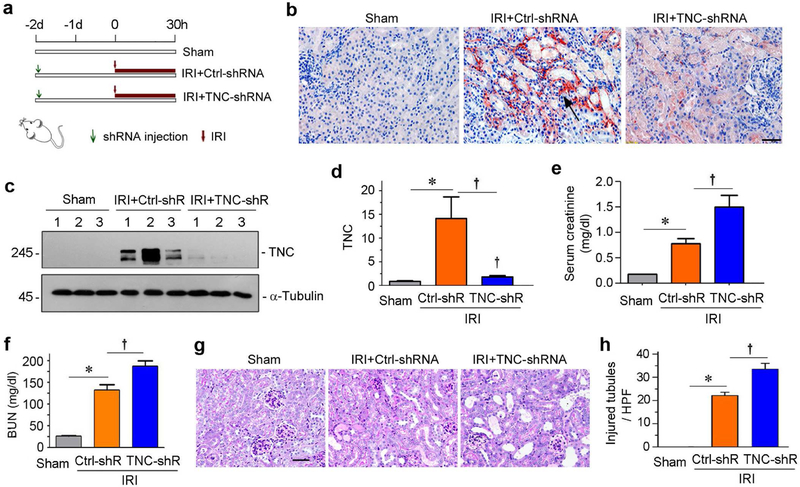
To investigate the function of TNC in AKI, we knocked down renal TNC expression in vivo by short hairpin RNA (shRNA)-mediated strategy. To this end, mice were intravenously injected with shRNA vector encoding the interference sequence of TNC (pLVX-shTNC) through a hydrodynamic-based gene delivery approach. Two days after injection, mice were subjected to IRI and sacrificed at 30 hours after surgery (Figure 2a). The efficacy of TNC knockdown was confirmed by both immunohistochemistry (Figure 2b) and western blotting (Figure 2, c and d). To examine the sequel of TNC depletion in AKI, serum creatinine and blood urea nitrogen (BUN) were assessed at 30 hours post-IRI. As shown in Figure 2, e and f, serum creatinine and BUN were elevated after IRI, and knockdown of TNC further increased their levels. We further examined renal histologic injury and quantified the injured tubules after periodic acid-Schiff (PAS) staining. As shown in Figure 2, g and h, tubular injury, characterized by loss of brush border, tubular cell loss and cast formation, were apparent after IRI, and knockdown of TNC further aggravated these tubular lesions.
Figure 2. Knockdown of TNC deteriorates kidney injury after IRI.
(a) Experimental design. Green arrows indicate the injection of pLVX-shTNC plasmid. Red arrows indicate the timing of IRI surgery. (b) Representative micrographs show renal TNC expression in different groups as indicated. Arrow indicates positive TNC expression. Scale bar, 40 μm. (c) Representative Western blot shows renal TNC protein expression in different groups as indicated. (d) Graphic presentation shows the relative TNC protein levels (fold induction over the controls) in different groups. *P < 0.05 versus controls; †P< 0.05 versus IRI injected with Ctrl-shRNA (n = 5 to 6). (e) Graphic presentation shows serum creatinine levels in different groups as indicated. *P < 0.05 versus controls; †P< 0.05 versus IRI injected with Ctrl-shRNA (n = 5 to 6). (f) Graphic presentation shows blood urea nitrogen (BUN) levels in different groups as indicated. *P < 0.05 versus controls; †P< 0.05 versus IRI injected with Ctrl-shRNA (n = 5 to 6). (g) Representative micrographs show kidney injury in different groups as indicated. Images of PAS staining were shown. Scale bar, 50 μm. (h) Graphic presentation shows the injured tubules per high power field (HPF) in three groups as indicated. *P< 0.05 versus controls; †P< 0.05 versus IRI injected with Ctrl-shRNA (n = 5 to 6).
TNC depletion aggravates tubular cell apoptosis
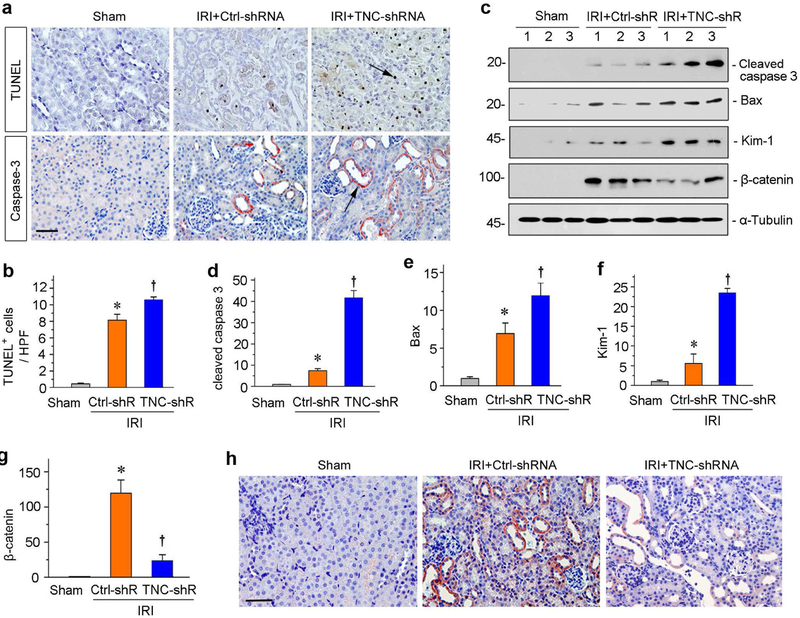
We next assessed apoptosis in the kidneys after various treatments. Apoptotic cells were detected by terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) staining. As shown in Figure 3a, IRI caused significant tubular cell apoptosis, and knockdown of TNC further exacerbated it. Quantitative data on apoptotic cells are presented in Figure 3b. Similarly, immunostaining for cleaved caspase 3 also demonstrated its upregulation in the kidney, and TNC depletion further increased its expression (Figure 3a). We then examined renal expression of apoptosis-related proteins by western blotting. As shown in Figure 3, c through e, cleaved caspase 3 and Bax were induced in the kidneys of IRI mice. However, TNC depletion further augmented their expression (Figure 3, c through e). Consistently, kidney injury molecule-1 (Kim-1), a tubular damage marker, was also upregulated after IRI, which was further enhanced after TNC depletion (Figure 3, c and f).
Figure 3. Knockdown of TNC aggravates tubular cell apoptosis and blocks β-catenin activation after IRI.
(a) Representative micrographs show TUNEL-positive cells and cleaved caspase-3 expression in different groups as indicated. Arrows indicate positive staining. Scale bar, 50 μm. (b) Graphic presentation shows the TUNEL-positive cells per high power field (HPF) in different groups as indicated. *P< 0.05 versus controls; †P< 0.05 versus IRI injected with Ctrl-shRNA (n = 5 to 6). (c) Representative Western blot analyses show renal expression of the cleaved caspase-3, Bax, Kim-1 and β-catenin proteins in different groups as indicated. Numbers (1, 2 and 3) indicate each individual animal in a given group. (d-g) Graphic presentations show the relative abundances of renal cleaved caspase-3 (d), Bax (e), Kim-1 (f) and β-catenin (g) proteins in different groups as indicated. *P < 0.05 versus controls; †P< 0.05 versus IRI injected with Ctrl-shRNA (n = 5 to 6). (h) Representative micrographs show β-catenin protein expression in different groups as indicated. Arrow indicates positive staining. Scale bar, 50 μm.
TNC depletion phenocopies tubular ablation of β-catenin
Earlier studies show that tubule-specific ablation of β-catenin aggravates kidney injury after IRI,12 reminiscent of TNC depletion in this setting. This prompted us to examine a possible connection between TNC and β-catenin. To this end, we examined renal expression of β-catenin by western blotting. As shown in Figure 3, c and g, β-catenin was markedly induced in the kidney after AKI, but knockdown of TNC largely blocked its induction. We further detected β-catenin by immunostaining. As shown in Figure 3h, β-catenin protein was clearly induced after IRI, particularly in the degenerated tubules with diluted lumens. However, TNC depletion inhibited its expression. These results suggest a close correlation between TNC and β-catenin activation in mediating renal protection against AKI.
TNC depletion exacerbates nephrotoxic AKI induced by cisplatin
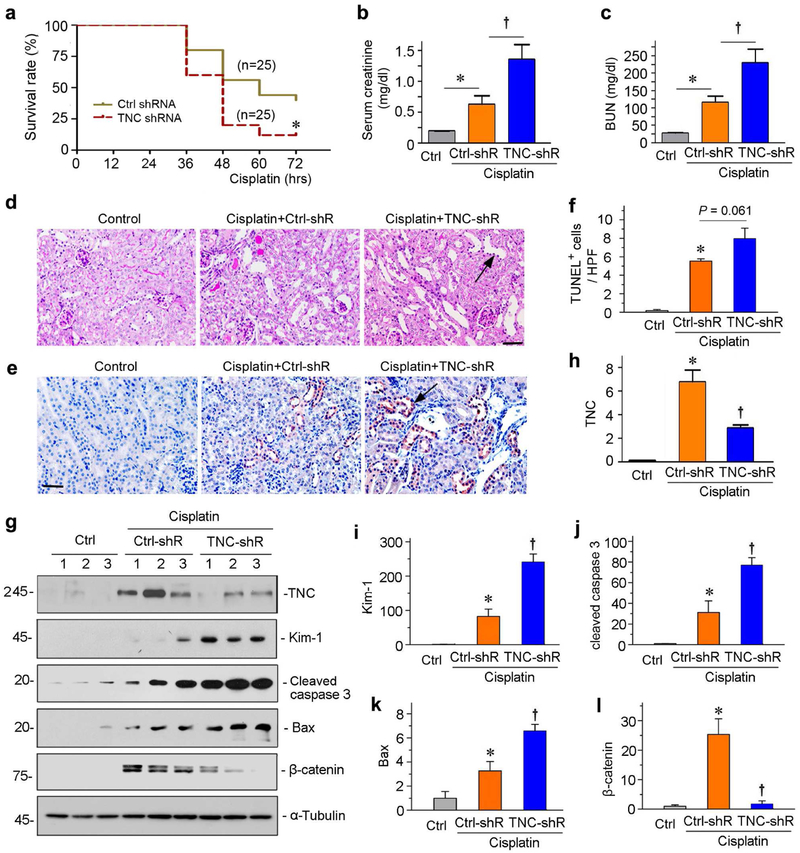
To further define the role of TNC in AKI, we adopted a nephrotoxic AKI model induced by cisplatin. As shown in Figure 4a, TNC deprivation increased the mortality rate of mice after cisplatin injection. Within 3 days after cisplatin, 88% of mice died in the group received TNC-shRNA, whereas 40% of mice survived in the same period in control group. These data suggest that TNC is renal protective and promotes survival after nephrotoxic injury.
Figure 4. Knockdown of TNC aggravates kidney injury in cisplatin-induced AKI.
(a) Knockdown of TNC increases animal mortality rate after cisplatin. *P< 0.05 (n= 25). (b, c) Graphic presentations show serum creatinine (b) and blood urea nitrogen (BUN) (c) levels in different groups as indicated at 36 hours after cisplatin injection. *P < 0.05 versus controls; †P< 0.05 versus Ctrl-shRNA (n = 5 to 6). (d) Representative micrographs show kidney injury in different groups at 36 hours after cisplatin injection. Images of PAS staining were shown. Arrow indicates the injured tubule. Scale bar, 50 μm. (e) Immunohistochemical staining shows cleaved caspase-3 protein expression in different groups as indicated. Arrow indicates positive staining. Scale bar, 50 μm. (f) Graphic presentations show TUNEL-positive cells in different groups as indicated. *P < 0.05 versus controls; †P< 0.05 versus cisplatin injected with CtrlshRNA (n = 5 to 6). (g) Representative Western blot analyses show renal expression of TNC, Kim-1, cleaved caspase-3, Bax and β-catenin expression in different groups as indicated. (h-l) Graphic presentations show the relative levels of TNC (h), Kim-1 (i), cleaved caspase-3 (j), Bax (k) and β-catenin (l) protein expressions in different groups as indicated. *P < 0.05 versus controls; †P< 0.05 versus cisplatin injected with Ctrl-shRNA (n = 5 to 6).
We carried out another experiment by sacrificing mice at 36 hours after cisplatin, a time point prior to animal loss (Figure 4a). As shown in Figure 4, b and c, depletion of TNC substantially increased serum creatinine and BUN in mice after injection of cisplatin, suggesting that endogenous TNC is renal protective in this model. In cisplatin-injected mice, there were focal sites of tubular injury characterized by loss of brush border, tubular dilation and cell death, and TNC depletion aggravated these lesions (Figure 4d). Furthermore, TNC depletion induced cleaved caspase 3 expression in cisplatin-injected kidneys (Figure 4e). To quantitatively assess apoptosis, we counted the numbers of apoptotic cells after TUNEL staining. As shown in Figure 4f, knockdown of TNC further increased tubular cell apoptosis in cisplatin-treated mice. Western blotting confirmed that depletion of TNC by this shRNA approach was effective (Figure 4, g and h), and TNC depletion increased renal expression of Kim-1, cleaved caspase 3 and Bax (Figure 4, g through k).
To validate the association of TNC with β-catenin, we examined renal expression of β-catenin in this model. Similar to IRI, β-catenin was upregulated in the kidney after cisplatin injection, and knockdown of TNC largely blocked its induction (Figure 4, g and l).
TNC prevents tubular cells apoptosis in vitro
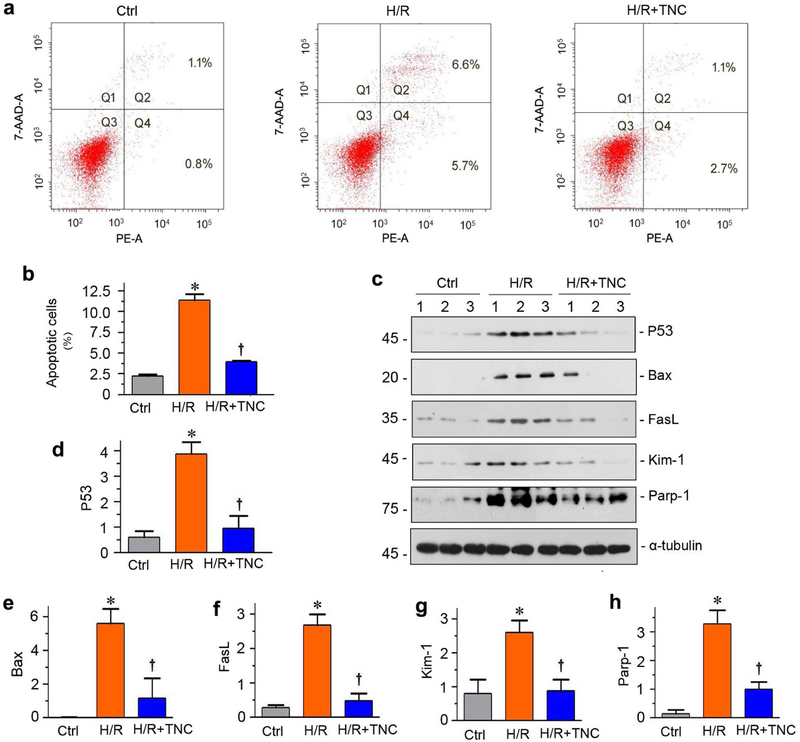
To directly confirm a role of TNC in protecting against apoptosis, we investigated the potential role of TNC in tubular cell survival after injury by using in vitro system. To mimic IRI in vitro, we treated human proximal tubular epithelial cells (HKC-8) with hypoxia/reoxygenation (H/R) injury. As shown in Figure 5, a and b, H/R triggered tubular cell apoptosis in vitro. However, co-incubation with TNC reduced H/R-mediated apoptosis, as assessed by Annexin V-labeling flow cytometry. H/R also up-regulated P53, Bax, Fas ligand (FasL) and Poly (ADP-ribose) polymerase 1 (Parp-1), as well as Kim-1, but these inductions were hampered after co-incubation with TNC (Figure 5, c through h).
Figure 5. TNC protects kidney tubular cells against apoptosis induced by hypoxia/reoxygenation injury in vitro.
(a) Representative FACS analyses show that TNC reduced hypoxia/reoxygenation (H/R)-induced cell apoptosis. HKC-8 cells were pre-incubated with recombinant TNC (50 ng/ml) for 1 hour, followed by incubation in hypoxic condition for 24 hours and then reoxygenation for 2 hours. (b) Graphic presentation shows the percentage of apoptotic cells in different groups as indicated. HKC-8 cells were treated with H/R injury in the absence or presence of TNC. The PE-labeled Annexin V-positive cells were counted by flow cytometry. *P < 0.05 versus controls; †P< 0.05 versus H/R (n = 3). (c) Representative Western blot analyses show protein expression of P53, Bax, FasL, Kim-1 and Parp-1 after various treatments in HKC-8 cells. (d-h) Graphic presentations show the relative abundances of P53 (d), Bax (e), FasL (f), Kim-1 (g) and Parp-1 (h) proteins in different groups as indicated. *P < 0.05 versus controls; †P< 0.05 versus H/R (n = 3).
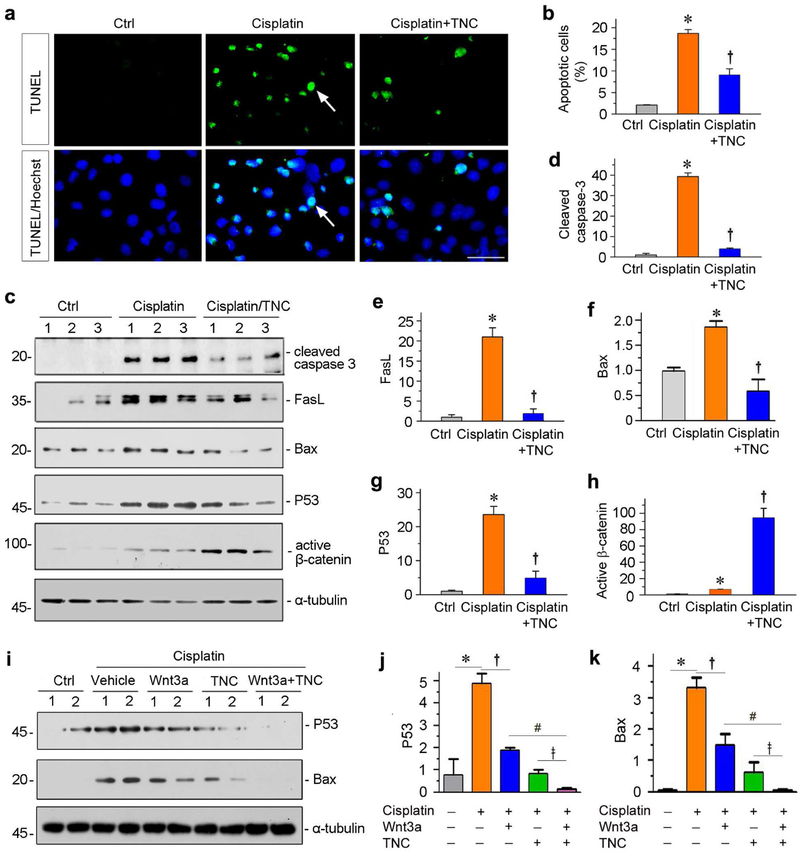
We also examined tubular cell apoptosis induced by cisplatin in the absence or presence of TNC. As shown in Figure 6a, substantial apoptosis was detected in HKC-8 cells by TUNEL staining after cisplatin treatment, but TNC inhibited it. Consistently, TNC decreased the percentage of apoptotic cells by flow cytometry (Figure 6b). We then assessed multiple apoptosis-regulatory proteins by western blotting. As shown in Figure 6, c through g, cleaved caspase 3, FasL, Bax and P53 were induced by cisplatin, but largely blocked by co-incubation with TNC. Similar results were obtained when we analyzed the role of TNC in preventing staurosporine-induced HKC-8 cell apoptosis (Supplementary Figure S1).
Figure 6. TNC protects kidney tubular cells against apoptosis induced by cisplatin in vitro.
(a) Representative micrographs show TUNEL-positive cells in different groups as indicated. Human kidney proximal tubular epithelial cells (HKC-8) were pre-incubated with recombinant TNC (50 ng/ml) for 1 hour, followed by incubating with cisplatin (25 μg/ml) for 36 hours. Arrows indicate TUNEL-positive apoptotic cells. (b) Graphic presentation shows the percentage of apoptotic cells in different groups as indicated. HKC-8 cells were treated with cisplatin in the absence or presence of TNC. The PE-labeled Annexin V-positive cells were counted by flow cytometry. *P < 0.05 versus controls; †P< 0.05 versus cisplatin alone (n = 3). (c) Representative Western blot analyses show protein expression of cleaved caspase-3, FasL, Bax, P53 and active β-catenin after various treatments in HKC-8 cells. (d-h) Graphic presentations show the relative abundances of cleaved caspase-3 (d), FasL (e), Bax (f), P53 (g) and active β-catenin (h) protein expressions in different groups as indicated. *P < 0.05 versus controls; †P< 0.05 versus cisplatin alone (n = 3). (i) Representative Western blot analyses show that TNC potentiated Wnt3a-mediated down-regulation of P53 and Bax expression induced by cisplatin. (j, k) Graphic presentations show the relative abundances of P53 (j) and Bax (k) protein expressions in different groups as indicated. *P < 0.05 versus controls; †P< 0.05 versus cisplatin alone; #P < 0.05 versus cisplatin plus Wnt3a; ǂ P< 0.05 versus cisplatin plus TNC (n = 3).
To delineate the mechanism of TNC in cell protection, we examined the expressions of active β-catenin. As shown in Figure 6, c and h, cisplatin slightly increased the expression of active β-catenin. However, co-incubation with TNC further upregulated the expression active β-catenin, suggesting that TNC-mediated cytoprotection is presumably operated through augmenting β-catenin signaling. Consistently, TNC was able to potentiate Wnt3a-mediated suppression of P53 and Bax in HKC-8 cells after cisplatin treatment (Figure 6, i through k).
TNC augments β-catenin signaling by binding to and presenting Wnt ligands
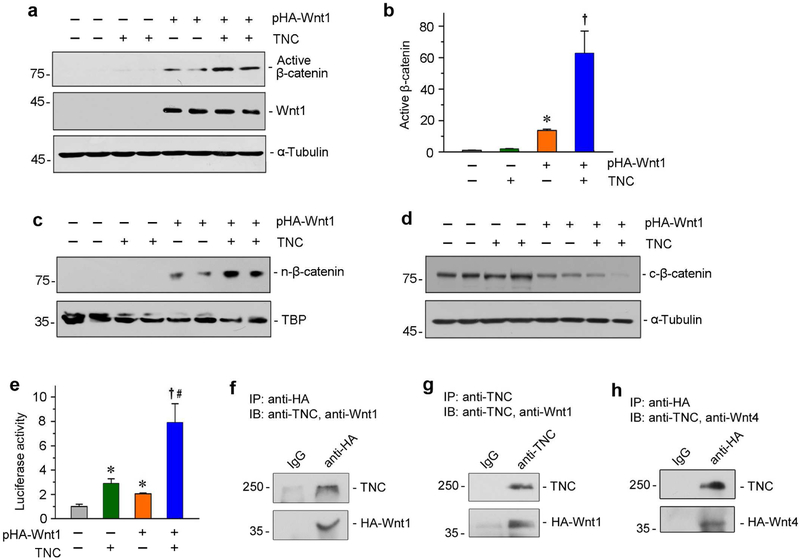
The connection between TNC and β-catenin prompted us to investigate the details of their interaction. To this end, we transfected HKC-8 cells with Wnt1 expression vector (pHA-Wnt1), followed by treatment with TNC protein. As shown in Figure 7, a and b, expression of Wnt1 induced β-catenin activation. Interestingly, incubation of TNC further augmented β-catenin activation, although similar level of Wnt1 was detected (Figure 7a). To further corroborate this finding, we next examined the subcellular localization of β-catenin after fractionation. As shown in Figure 7c, Wnt1 expression induced nuclear accumulation of β-catenin, and TNC further promoted its nuclear translocation. Conversely, cytoplasmic β-catenin was decreased after transfection with Wnt1, which was further reduced by TNC (Figure 7d). To study the functional sequel of TNC on Wnt/β-catenin, we assessed the Wnt-mediated gene transcription in a β-catenin responsive TOP-Flash luciferase reporter assay. As shown in Figure 7E, transfection of Wnt1 expression vector induced luciferase activity, as expected. However, incubation with TNC markedly augmented Wnt1-mediated reporter gene expression (Figure 7e).
Figure 7. TNC potentiates Wnt/β-catenin signaling by physically interacting with Wnt ligands.
(a, b) Western blot analyses show that TNC augmented Wnt1-triggered β-catenin activation. HKC-8 cells were transfected with Wnt1 expression vector (pHA-Wnt1) or empty vector in the absence or presence of TNC as indicated. Whole cell lysates were analyzed by Western blotting with specific antibodies against dephosphorylated, active β-catenin, or Wnt1. Representative Western blot (a) and graphic presentation of active β-catenin abundance (b) were presented. *P < 0.05 versus empty vector controls (n = 3); †P< 0.05 versus pHA-Wnt1 alone (n = 3). (c, d) TNC augmented Wnt1-triggered β-catenin nuclear translocation. Nuclear (c) and cytoplasmic (d) proteins were prepared from HKC-8 cells after various treatments as indicated, and immunoblotted with antibodies against β-catenin. Nuclear β-catenin (n-β-catenin) and cytoplasmic β-catenin (c-β-catenin) were normalized with TATA-binding protein (TBP) and α-tubulin, respectively. (e) TNC promotes β-catenin-driven gene transcription. HKC-8 cells were transfected with TOP-Flash reporter plasmid and pHA-Wnt1 plasmid in the absence or presence of TNC. TOP-Flash reporter luciferase activities were assessed, and relative luciferase activity (fold induction) was reported. *P < 0.05 versus empty vector controls (n = 3); †P< 0.05 versus pHA-Wnt1 alone (n = 3). (f-h) Coimmunoprecipitation (Co-IP) demonstrates that TNC bound to Wnt1 and Wnt4 in tubular epithelial cells. HKC-8 cells were transfected with HA-tagged Wnt1 (pHA-Wnt1) or Wnt4 expression vectors (pHA-Wnt4), followed by incubation with TNC. Cell lysates were immunoprecipitated with anti-HA or anti-TNC, followed by immunoblotting with anti-TNC, anti-Wnt1 or anti-Wnt4, respectively.
To elucidate how TNC potentiates Wnt/β-catenin signaling, we investigated the potential interaction between TNC and Wnt ligands. HKC-8 cells were transfected with pHA-Wnt1, followed by incubation with TNC. As shown in Figure 7f, TNC was detectable in the complexes precipitated with anti-HA antibody. In the reciprocal experiments, Wnt1 was observed in the complexes precipitated with anti-TNC antibody (Figure 7g). Similar results were obtained when HKC-8 cells were transfected with pHA-Wnt4, followed by immunoprecipitation (Figure 7h). There results indicate that TNC can physically interact with Wnt ligands by forming a complex.
TNC recruits and concentrates Wnt ligands ex vivo
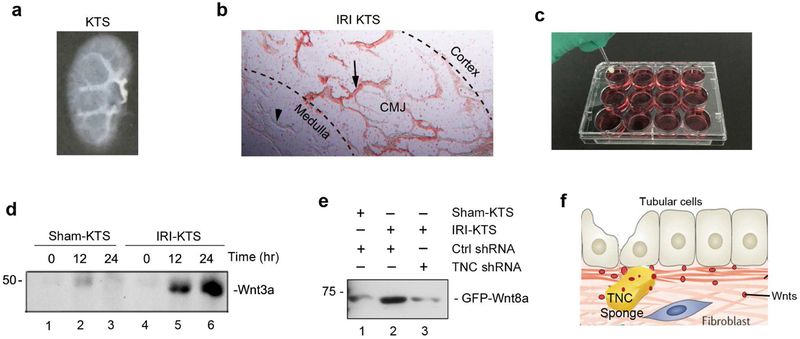
Because TNC binds to Wnt ligands, we speculated that TNC might be able to recruit Wnt ligands from its surrounding extracellular mellitus to build up a special niche with a high level of Wnts. To test this hypothesis, we prepared the decellularized kidney tissue scaffold (KTS) from IRI kidneys (IRI-KTS) which was rich in TNC, by procedures as reported recently.20 Figure 8a shows a typical KTS, in which no cell was found after staining for the nucleus with DAPI (data not shown). Abundant TNC staining was evident in the junctional region of renal cortex and medulla in IRI-KTS (Figure 8b). We then placed the KTS prepared from sham control (sham-KTS) and IRI kidneys to culture wells containing medium supplemented with purified Wnt3a (Figure 8c). After incubation for various periods of time as indicated, both sham-KTS and IRI-KTS were collected and lysed, and Wnt3a abundance in KTS was analyzed by Western blotting. As shown in Figure 8d, Wnt3a was clearly enriched in a time-dependent manner only in IRI-KTS, but not in sham-KTS. To further confirm the role of TNC in recruiting Wnt ligands, we knocked down renal TNC expression by injecting mice with pLVX-shTNC plasmid, which was followed by IRI. We then prepared KTS from different groups of mice, and incubated with the medium containing GFP-Wnt8a fusion protein. As presented in Figure 8e, GFP-Wnt8a fusion protein was greatly enriched in the IRI-KTS, compared to the sham-KTS. However, when TNC was depleted by shRNA, little GFP-Wnt8a was accumulated in the IRIKTS (Figure 8e, lane 2 versus lane 3). As shown in Figure 8f, these results suggest that TNC is able to recruit Wnt ligands from surrounding extracellular mellitus, thereby creating a unique tissue microenvironment in which high level of Wnts are present.
Figure 8. TNC-enriched kidney tissue scaffold (KTS) recruits Wnt ligands.
(a) Decellularized KTS. (b) Micrograph shows TNC-positive region in the IRI-KTS. Arrow indicates TNC-positive region, whereas arrowhead denotes TNC-negative area. CMJ, corticomedullary junction. (c) Experimental design for testing TNC recruiting Wnt ligands. Sham-KTS or IRI-KTS were placed in medium containing Wnt3a or GFPWnt8a fusion protein, and incubated for various periods of time as indicated. (d) TNC-enriched IRI-KTS recruits Wnt3a from surrounding medium. Sham-KTS or IRI-KTS were incubated in the medium containing recombinant Wnt3a for different time periods as indicated, followed by Western blotting with anti-Wnt3a antibody. (e) TNC is required for IRI-KTS recruiting Wnt ligands. KTS was prepared from different groups as indicated, and incubated in the medium containing GFP-Wnt8a fusion protein, then followed by Western blotting. (f) Diagram shows that TNC, acting as a sponge, recruits and concentrates Wnt ligands from surrounding extracellular mellitus. This creates a unique tissue microenvironment in which Wnt ligands are enriched and presented to the responsive cells.
DISCUSSION
Despite intensive studies in last several decades, the mortality rate of patients with severe AKI remains distressingly high.3 This reality calls for a conceptual change in our understanding of the patho-mechanisms behind AKI. While tubular injury, endothelial dysfunction and inflammation are well known for their involvement in AKI, the impact of tissue microenvironment on the outcome of AKI was largely overlooked in the past. In the present study, we demonstrate that a TNC-rich niche is crucial for tubular cell survival and recovery after AKI. TNC is specifically induced and localizes locally in the injurious sites of the kidneys, and it binds to Wnt ligands. As such, TNC is able to recruit and concentrate Wnt ligands from the surrounding milieu and present them to neighboring cells, which creates a favorable microenvironment for a constructive response of tubular cells after injury (Figure 8). These findings, for the first time, underscore a pivotal role of tissue microenvironment in conferring renal protection against acute injury.
TNC is an ECM-binding glycoprotein with multiple biologic functions, and plays an essential role in regulating cell survival, proliferation, migration and matrix assembly.18, 24, 29 As a scaffold protein, TNC is known to be instrumental in constructing a propitious niche for stem or progenitor cells.19 In the setting of CKD, TNC is involved in the formation of a pro-fibrotic niche, in which fibroblast cells are prone to activation and proliferation after stimulation.20 Of interest, TNC is also induced in acute phase of kidney injury (Figure 1), suggesting a potential role in the development of AKI. Whether de novo induction of TNC is beneficial or detrimental to the kidneys in AKI was unknown. In this context, it is both important and timely to delineate the functional role of TNC in AKI and to elucidate the underlying mechanisms.
The study presented here highlights that TNC is a key player in preventing tubular injury after AKI by building a reparative microenvironment. This conclusion is supported by several lines of evidence. The early induction of TNC occurs in AKI induced by IRI or cisplatin, as well as in human kidney biopsies, and it specifically localizes in the injurious sites (Figure 1). Moreover, the circulating level of TNC is elevated in AKI patients at 48 hours after cardiac surgery, and the magnitude of its induction is closely correlated with the severity of kidney injury (Figure 1). The cellular sources of TNC protein are likely to be interstitial fibroblasts,20 although this issue is not specifically addressed in this study. We show that knockdown of TNC by shRNA-mediated strategy aggravates tubular cell apoptosis, kidney injury and animal death, indicating that TNC induction after AKI is renal protective. Mechanistically, TNC is able to potentiate Wnt/β-catenin signaling, a developmental signaling well known to protect kidneys against AKI.12, 30, 31 Taken together, our findings provide unambiguous evidence that the de novo induction of TNC is an initial attempt of damaged kidney to protect against injury and promote recovery after AKI.
The most novel and interesting finding of the present study is that TNC possesses a unique ability to recruit and concentrate Wnt ligands from surrounding milieu, thereby creating a specialized microenvironment in which Wnts are enriched. This speculation is confirmed by an ex vivo approach using TNC-rich KTS (Figure 8). The decellularized IRI-KTS realistically imitates in vivo tissue environment in which TNC is present in renal corticomedullary junctional area (Figure 8b), and is able to recruit Wnt proteins from the surrounding medium. Notably, TNC is a large hexameric protein that can potentially bind to multiple Wnts at the same time,24 and therefore could possess a tremendous ability to recruit Wnt ligands. It is conceivable to propose that TNC functions as a ‘molecular sponge’ that attracts and recruits Wnt ligands from the surrounding microenvironment (Figure 8).
The connection between TNC and Wnt/β-catenin signaling is further supported by in vivo and in vitro evidence. Mice with TNC depletion display a reduced β-catenin activation (Figure 3 and 4) and phenocopy the mice with tubule-specific deletion of β-catenin after AKI.12 Consistently, TNC potentiates β-catenin activation in HKC-8 cells in vitro, despite of a constant level of Wnt1 expression (Figure 7); and it promotes Wnt3a-mediated cytoprotection against cisplatin (Figure 6). Furthermore, we demonstrate the physical interaction of TNC with Wnt1 and Wnt4 ligands (Figure 7) and recruitment of Wnt3a and Wnt8a (Figure 8), which is consistent with earlier report that TNC could be co-immunoprecipitated with Wnt3a.32 Therefore, it is plausible that TNC recruits and retains Wnt ligands through physical interaction, thereby building up a microenvironment in which a high level of Wnt ligands is present.
It is worthwhile to point out that TNC may promote Wnt/β-catenin signaling through other mechanisms as well. In this regard, earlier studies showed that TNC downregulates Wnt inhibitor Dickkopf-1 in tumor cells,33 suggesting another possibility for TNC to create a permissive microenvironment for Wnt signaling. Furthermore, TNC could function as a ligand to activate integrin αVβ3, which evokes phosphorylation of focal adhesion kinase (FAK) and Src non-receptor tyrosine kianse.20, 34, 35 Both FAK and Src are involved in activation of Wnt/Frizzeled/LRP complex and nuclear localization of β-catenin.36–38 Therefore, the connection between TNC and Wnt/β-catenin could be operated through multiple routes. More future studies are needed in this area.
The renoprotective role of TNC in AKI, as presented here, is utterly different from its detrimental action in CKD.20 At first glance, this seems paradoxical and contradictory. However, the ability of TNC to recruit Wnt ligands well explains for its conflicting actions in AKI versus CKD. It has been demonstrated that Wnt/β-catenin is protective in AKI by promoting tubular cell survival and proliferation,12, 30, 39 but sustained activation of this signaling in CKD leads to tubular impairment, myofibroblast activation and renal fibrosis by a multitude of mechanisms.7, 40, 41 Therefore, by creating Wnts-enriched microenvironment, TNC renders opposite effects on the kidney in the settings of AKI versus CKD through the same mechanism. It is conceivable that if TNC induction is exaggerated or sustained after severe or repeated AKI, it could play an important role in mediating AKI-to-CKD continuum by virtue of its ability to recruit Wnts. Consistent with this, depletion of TNC at a later time point such as 10 days after IRI would hinder progression to CKD.
In summary, we have shown that TNC, an ECM protein, plays an important role in protecting kidneys against AKI. This beneficial effect of TNC is largely mediated by its ability to recruit and retain Wnt ligands, thereby creating a favorable microenvironment for protecting tubular cells from injury. These findings provide a novel mechanistic link among TNC, Wnt/β-catenin and tubular cell protection. Our results also suggest a previously unrecognized role of tissue microenvironment in regulating kidney injury and repair in AKI.
CONCISE METHODS
Mouse models of AKI
Male C57BL/6 mice were obtained from the Southern Medical University Animal Center (Guangzhou, China). For ischemic AKI, bilateral renal pedicles were clipped for 30 minutes in mice using microaneurysm clamps, using an established protocol.12 Mice were sacrificed at 30 hours post-IRI and blood and tissue samples collected. Pilot experiments showed that renal injury and dysfunction reached the peak at 30 hours after bilateral IRI, and animals started to die in this model.
For toxic AKI, mice were subjected to a single intraperitoneal injection of cisplatin (Sigma, St. Louis, MO). Cisplatin was administered at a dose of 40 mg/kg as described elsewhere.42 For animal survival experiments, 25 mice were used in each group and survival of animals monitored for 72 hours. In another set of experiment, mice were sacrificed at 36 hours after cisplatin injection. All animal experiments were approved by the Animal Ethic Committee at the Nanfang Hospital, Southern Medical University.
Human Samples
All studies involving human samples were approved by the Ethic Committee on Human Subjects at the Nanfang hospital, Sothern Medical University. The study participants provided written informed consent. Healthy subjects (26 cases) were recruited and blood collected. Patients receiving elective cardiac surgery who developed AKI were also recruited. Among them, 30 patients developed severe AKI, and 30 patients developed mild AKI. Plasma samples were obtained at 48 hours after surgery. Exclusion criteria included exposure to nephrotoxin (i.e., contrast media, aminoglycoside antibiotics, vancomycin, and nonsteroidal anti-inflammatory drugs except aspirin) within 4 weeks before surgery, preexisting advanced CKD (chronic dialysis, renal transplantation, or preoperative eGFR<30 ml/min per 1.73 m2), and urinary tract infection or obstruction. The demographic and clinical data of the healthy subjects and patients were presented in Supplementary Table 1. Human kidney biopsy sections from AKI patients (2 cases) were also obtained from diagnostic renal biopsies performed at the Nanfang Hospital and used for immunostaining for TNC.
TNC ELISA
Human Tenascin-C Assay Kit was purchased from the Immuno-Biological Laboratories. This assay is a solid phase sandwich ELISA using two kinds of specific antibodies for human TNC. Plasma TNC expressed as nanograms per milliliter.
Knockdown of TNC in vivo
Knockdown of TNC expression in vivo was carried out by shRNA-mediated approach. Mice were divided into three groups as indicated. Two days prior to IRI, mice were injected with plasmids via tail vein using hydrodynamic-based gene delivery technique, as described previously.43 Mice were then subjected to IRI. Animals were sacrificed at 30 h after IRI. Similar approach was used to knockdown TNC expression in cisplatin model in vivo. Mice were sacrificed at 36 h after cisplatin injection.
Determination of serum creatinine and BUN
Serum creatinine and blood urea nitrogen (BUN) were determined by an automatic chemistry analyzer. The levels of serum creatinine and BUN were expressed as mg/dl.
Cell culture and treatment
Human proximal tubular epithelial cells (HKC-8) were described previously,44 and treated with cisplatin or staurosporine at the specified concentrations. For some experiments, cells were pretreated with TNC at 50 ng/ml for 1 hour. Cells were analyzed with PE-conjugated Annexin V (AV)-labeled apoptotic cells detection kit, followed by analyzing with flow cytometry.
Hypoxia/reoxygenation injury
Hypoxia/reoxygenation injury was used to mimic IRI in vitro. Briefly, cells were incubated in glucose-free medium in a tri-gas incubator (94% N2, 5% CO2 and 1.0% O2) at 37 °C for 24 hours. Subsequently, cells were incubated in complete medium under normal conditions for 2 hours for reoxygenation.
TUNEL assays
TUNEL staining for apoptotic cells was performed on the paraffin-embedded mouse kidney sections (4 μm thickness) using a standard DAB incorporation kit according to the instructions specified by the manufacturer.42 HKC-8 cells were cultured on the coverslips and subjected to TUNEL staining.
Western blot analysis
Protein expression was analyzed by Western blot analysis as described previously.20 The primary antibodies used were described in the Supplementary Detailed Methods.
Reverse transcriptase (RT)-PCR
Total RNA was prepared using TRIzol RNA isolation system according to the manufacturer’s instruction. PCR amplification was performed using a HotStar Taq Master Mix kit, as described previously.43
Histology and immunohistochemical staining
Paraffin-embedded mouse kidney sections were prepared by a routine procedure. Immunohistochemical staining was performed using routine protocol. Antibodies used were described in the Supplementary Detailed Methods.
Immunoprecipitation
The interaction of TNC and Wnts was determined by co-immunoprecipitation, as previously described.44 HKC-8 cells were transfected with different HA-tagged Wnt expression plasmids (pHA-Wnt1 or pHA-Wnt4) and incubated in the absence or presence of TNC for 24 hours. Cell lysates were immunoprecipitated overnight at 4°C with anti-TNC antibody and protein A/G plus agarose. In the reciprocal experiments, cell lysates were immunoprecipitated with anti-HA antibody, followed by immunoblotting with anti-TNC antibody.
Luciferase Assay
The effect of TNC on Wnt-mediated gene transcription was assessed by using the TOP-Flash reporter. HKC-8 cells were transfected using Lipofectamine 2000 reagent. Luciferase assay was performed using a dual luciferase assay system kit. Relative luciferase activity was reported as fold induction over the controls after normalizing for transfection efficiency.
Nuclear and Cytoplasmic Fractionation
Nuclear and cytoplasmic protein was prepared using a commercial kit according to the protocols specified by the manufacturer.
Preparation of kidney tissue scaffold
Kidney tissue scaffolds (KTS) were prepared from sham controls or ischemic kidneys at 30 hours after IRI, according to protocols reported previously.20
Statistical analyses
All data examined were expressed as mean ± SEM. Statistical analysis of the data was carried out using SPSS 13.0. Comparison between groups was made using one-way ANOVA followed by Student-Newman-Kuels test or Dunnett’s T3 procedure. The animal survival curve was analyzed by Log-rank test. P < 0.05 was considered significant.
Supplementary Material
Figure S1. TNC protects kidney tubular cells against apoptosis induced by staurosporine in vitro. (a) Representative FACS analyses show that TNC reduced staurosporine-induced cell apoptosis. HKC-8 cells were pre-incubated with recombinant TNC (50 ng/ml) for 1 hour, followed by incubating with staurosporine (1 μM) for 6 hours. (b) Graphic presentation shows the percentage of apoptotic cells in different groups as indicated. HKC-8 cells were treated with staurosporine in the absence or presence of TNC. The PE-labeled Annexin V-positive cells were counted by flow cytometry. *P < 0.05 versus controls; †P < 0.05 versus staurosporine alone (n = 3). (c) Representative Western blot analyses show protein expression of the cleaved caspase-3, Bax, FasL and survivin after various treatments in HKC-8 cells. (d-g) Graphic presentations show the relative abundances of cleaved caspase-3 (d), Bax (e), FasL (f) and survivin (g) proteins in different groups as indicated. *P < 0.05 versus controls; †P < 0.05 versus staurosporine alone (n = 3).
Supplementary material: DETAILED METHODS
Supplementary Table 1. Demographic and clinical data of healthy subjects and AKI patients after cardiac surgery
ACKNOWLEDGEMENTS
This work was supported by National Natural Science Foundation of China Grant 81521003, 81770737, 81722011, and Guangdong Science Foundation grant 2014A030312014, and National Institute of Health grant DK064005 and DK091239.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
All the authors declared no competing interests.
REFERENCES
- 1.Thongprayoon C, Cheungpasitporn W, Akhoundi A, et al. Actual versus ideal body weight for acute kidney injury diagnosis and classification in critically ill patients. BMC Nephrol 2014;15:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanmassenhove J, Kielstein J, Jorres A, et al. Management of patients at risk of acute kidney injury. Lancet 2017;389:2139–2151. [DOI] [PubMed] [Google Scholar]
- 3.Odutayo A, Wong CX, Farkouh M, et al. AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol 2017;28:377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewington AJ, Cerda J, Mehta RL. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int 2013;84:457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X, Nie S, Liu Z, et al. Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol 2015;10:1510–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carney EF. Transplantation: New tool for prediction of ESRD risk in living kidney donor candidates. Nat Rev Nephrol 2016;12:1. [DOI] [PubMed] [Google Scholar]
- 7.Xiao L, Zhou D, Tan RJ, et al. Sustained activation of Wnt/beta-catenin signaling drives AKI to CKD progression. J Am Soc Nephrol 2016;27:1727–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 2011;7:189–200. [DOI] [PubMed] [Google Scholar]
- 9.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 2011;121:4210–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang D, Liu Y, Wei Q, et al. Tubular p53 regulates multiple genes to mediate AKI. J Am Soc Nephrol 2014;25:2278–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi M, Flores B, Gillings N, et al. alphaKlotho Mitigates Progression of AKI to CKD through Activation of Autophagy. J Am Soc Nephrol 2016;27:2331–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou D, Li Y, Lin L, et al. Tubule-specific ablation of endogenous beta-catenin aggravates acute kidney injury in mice. Kidney Int 2012;82:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulay SR, Kumar SV, Lech M, et al. How Kidney Cell Death Induces Renal Necroinflammation. Semin Nephrol 2016;36:162–173. [DOI] [PubMed] [Google Scholar]
- 14.Kinsey GR. Macrophage dynamics in AKI to CKD progression. J Am Soc Nephrol 2014;25:209–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhaun N, Webb DJ. The road from AKI to CKD: the role of endothelin. Kidney Int 2013;84:637–638. [DOI] [PubMed] [Google Scholar]
- 16.Ramesh G, Zhang B, Uematsu S, et al. Endotoxin and cisplatin synergistically induce renal dysfunction and cytokine production in mice. Am J Physiol Renal Physiol 2007;293:F325–F332. [DOI] [PubMed] [Google Scholar]
- 17.Zhang B, Ramesh G, Uematsu S, et al. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J Am Soc Nephrol 2008;19:923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tucker RP, Chiquet-Ehrismann R. Tenascin-C: Its functions as an integrin ligand. Int J Biochem Cell Biol 2015;65:165–168. [DOI] [PubMed] [Google Scholar]
- 19.Chiquet-Ehrismann R, Orend G, Chiquet M, et al. Tenascins in stem cell niches. Matrix Biol 2014;37:112–123. [DOI] [PubMed] [Google Scholar]
- 20.Fu H, Tian Y, Zhou L, et al. Tenascin-C is a major component of the fibrogenic niche in kidney fibrosis. J Am Soc Nephrol 2017;28:785–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharyya S, Wang W, Morales-Nebreda L, et al. Tenascin-C drives persistence of organ fibrosis. Nat Commun 2016;7:11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imanaka-Yoshida K Tenascin-C in cardiovascular tissue remodeling: from development to inflammation and repair. Circ J 2012;76:2513–2520. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida T, Akatsuka T, Imanaka-Yoshida K. Tenascin-C and integrins in cancer. Cell Adh Migr 2015;9:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giblin SP, Midwood KS. Tenascin-C: Form versus function. Cell Adh Migr 2015;9:48–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Laporte L, Rice JJ, Tortelli F, et al. Tenascin C promiscuously binds growth factors via its fifth fibronectin type III-like domain. PLoS One 2013;8:e62076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piccinini AM, Zuliani-Alvarez L, Lim JM, et al. Distinct microenvironmental cues stimulate divergent TLR4-mediated signaling pathways in macrophages. Sci Signal 2016;9:ra86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Midwood K, Sacre S, Piccinini AM, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med 2009;15:774–780. [DOI] [PubMed] [Google Scholar]
- 28.von Holst A Tenascin C in stem cell niches: redundant, permissive or instructive? Cells Tissues Organs 2008;188:170–177. [DOI] [PubMed] [Google Scholar]
- 29.Kasprzycka M, Hammarstrom C, Haraldsen G. Tenascins in fibrotic disorders-from bench to bedside. Cell Adhesion & Migration 2015;9:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou D, Tan RJ, Fu H, et al. Wnt/beta-catenin signaling in kidney injury and repair: a double-edged sword. Lab Invest 2016;96:156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin SL, Li B, Rao S, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A 2010;107:4194–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hendaoui I, Tucker RP, Zingg D, et al. Tenascin-C is required for normal Wnt/beta-catenin signaling in the whisker follicle stem cell niche. Matrix Biol 2014;40:46–53. [DOI] [PubMed] [Google Scholar]
- 33.Saupe F, Schwenzer A, Jia Y, et al. Tenascin-C downregulates wnt inhibitor dickkopf-1, promoting tumorigenesis in a neuroendocrine tumor model. Cell Rep 2013;5:482–492. [DOI] [PubMed] [Google Scholar]
- 34.Shimojo N, Hashizume R, Kanayama K, et al. Tenascin-C may accelerate cardiac fibrosis by activating macrophages via the integrin alphaVbeta3/nuclear factor-kappaB/interleukin-6 axis. Hypertension 2015;66:757–766. [DOI] [PubMed] [Google Scholar]
- 35.Ishigaki T, Imanaka-Yoshida K, Shimojo N, et al. Tenascin-C enhances crosstalk signaling of integrin alphavbeta3/PDGFR-beta complex by SRC recruitment promoting PDGF-induced proliferation and migration in smooth muscle cells. J Cell Physiol 2011;226:2617–2624. [DOI] [PubMed] [Google Scholar]
- 36.Ridgway RA, Serrels B, Mason S, et al. Focal adhesion kinase is required for beta-catenin-induced mobilization of epidermal stem cells. Carcinogenesis 2012;33:2369–2376. [DOI] [PubMed] [Google Scholar]
- 37.Despeaux M, Chicanne G, Rouer E, et al. Focal adhesion kinase splice variants maintain primitive acute myeloid leukemia cells through altered Wnt signaling. Stem Cells 2012;30:1597–1610. [DOI] [PubMed] [Google Scholar]
- 38.Buelli S, Rosano L, Gagliardini E, et al. beta-arrestin-1 drives endothelin-1-mediated podocyte activation and sustains renal injury. J Am Soc Nephrol 2014;25:523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuncewitch M, Yang WL, Corbo L, et al. WNT agonist decreases tissue damage and improves renal function after ischemia-reperfusion. Shock 2015;43:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan RJ, Zhou D, Zhou LL, et al. Wnt/beta-catenin signaling and kidney fibrosis. Kidney Int Suppl 2014;4:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou LL, Li YJ, Hao S, et al. Multiple genes of the renin-angiotensin system are novel targets of Wnt/beta-catenin signaling. J Am Soc Nephrol 2015;26:107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou D, Tan RJ, Lin L, et al. Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int 2013;84:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou D, Li Y, Zhou L, et al. Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J Am Soc Nephrol 2014;25:2187–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou D, Tian Y, Sun L, et al. Matrix metalloproteinase-7 is a urinary biomarker and pathogenic mediator of kidney fibrosis. J Am Soc Nephrol 2017;28:598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. TNC protects kidney tubular cells against apoptosis induced by staurosporine in vitro. (a) Representative FACS analyses show that TNC reduced staurosporine-induced cell apoptosis. HKC-8 cells were pre-incubated with recombinant TNC (50 ng/ml) for 1 hour, followed by incubating with staurosporine (1 μM) for 6 hours. (b) Graphic presentation shows the percentage of apoptotic cells in different groups as indicated. HKC-8 cells were treated with staurosporine in the absence or presence of TNC. The PE-labeled Annexin V-positive cells were counted by flow cytometry. *P < 0.05 versus controls; †P < 0.05 versus staurosporine alone (n = 3). (c) Representative Western blot analyses show protein expression of the cleaved caspase-3, Bax, FasL and survivin after various treatments in HKC-8 cells. (d-g) Graphic presentations show the relative abundances of cleaved caspase-3 (d), Bax (e), FasL (f) and survivin (g) proteins in different groups as indicated. *P < 0.05 versus controls; †P < 0.05 versus staurosporine alone (n = 3).
Supplementary material: DETAILED METHODS
Supplementary Table 1. Demographic and clinical data of healthy subjects and AKI patients after cardiac surgery