Abstract
Aging results in increased activation of inflammatory glial cells and decreased neuronal viability following spinal cord injury (SCI). Metabolism and transport of glucose is also decreased with age, although the influence of age on glucose transporter (GLUT) expression or glucose uptake in SCI is currently unknown. We therefore performed [18F]Fluorodeoxyglucose (FDG) PET imaging of young (3 month) and middle-aged (12 month) rats. Glucose uptake in middle-aged rats was decreased compared to young rats at baseline, followed by increased uptake 14 days post contusion SCI. qRT-PCR and protein analysis revealed an association between 14 day glucose uptake and 14 day post-injury inflammation. Further, gene expression analysis of neuron-specific GLUT3 and non-specific GLUT4 (present on glial cells) revealed an inverse relationship between GLUT3/4 gene expression and glucose uptake patterns. Protein expression revealed increased GLUT3 in 3 month rats only, consistent with age related decreases in glucose uptake, and increased GLUT4 in 12 month rats only, consistent with age related increases in inflammatory activity and glucose uptake. Inconsistencies between gene and protein suggest an influence of age-related impairment of translation and/or protein degradation. Overall, our findings show that age alters glucose uptake and GLUT3/4 expression profiles before and after SCI, which may be dependent on level of inflammatory response, and may suggest a therapeutic avenue in addressing glucose uptake in the aging population.
Keywords: Aging, FDG-PET Imaging, Inflammation, Glucose metabolism, Glucose transporter, Spinal cord injury
Introduction
The average age of spinal cord injury (SCI) has been steadily increasing, with a current average age of onset of 42 [1,2]. Though SCI has largely been studied in the young adult population, the effect of aging on SCI has been receiving increased attention [3–6]. Cellular changes after SCI include acute neuron, astrocyte and oligodendrocyte reduction of cell viability, followed by chronic white matter and axon loss, glial scar formation, and inflammation [7].
Aged spinal cord tissue shows increased basal inflammatory activity and oxidative stress [4,5,8], resulting in reduced neuronal viability and contributing to worsened outcome following SCI [3,9–11]. Work in our lab and work from other groups has demonstrated that aging increases glial cell activity and promotes an exaggerated response to SCI [4–6,12–14].
Glucose is the major energy source for the central nervous system (CNS) [15,16]. Previous work in our lab using positron emission tomography (PET)-based measurements of glucose uptake demonstrated an acute reduction at 6 hours post-injury in young adult rats [17]. Aging results in impairments of glucose metabolism and transport in brain and fatty tissue [18–21], although the impact of aging on the post-SCI pattern of glucose uptake is currently unknown.
Glucose is transported into cells by glucose transporters (GLUTs) [15,22,23]. There are multiple isoforms of GLUTs, including GLUT3 (abundant in neurons) [24,25] and GLUT4 (insulin-sensitive and present on neurons, microglia, blood vessels and astrocytes) [25–28]. Both age and injury lead to alterations in GLUT functionality in the brain and skeletal muscle, which can contribute to impaired glucose uptake and metabolism [18–20,29].
The purpose of this study was to determine how age and its associated cellular changes alter the glucose uptake profile in the naïve and injured rodent spinal cord. We now show that age significantly reduces basal glucose uptake, which is associated with GLUT3 and 4 gene but not protein expression elevation, and that injury results in a marked elevation in glucose uptake at 14 dpi in middle-aged rats that is associated with elevation in inflammation.
Materials and Methods
Animals
Male Sprague Dawley rats were given free access to food and water and a 12h light/12h dark cycle. Young adult rats (3 months old: 300-500g; Harlan, Frederick, MD: n=32) were housed for 1 week prior to entering the study. Middle-aged rats (12 months old, retired breeders: 400-600g; Harlan, Frederick, MD: n=24) were acquired at 10 months of age, and gentled twice a week until 12 months of age. Division of animals by experimental group is outlined in Table 1. All animal procedures were approved by the Uniformed Services University IACUC and complied fully with the principles set forth in the “Guide for the Care and Use of Laboratory Animals” prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Resources, National Research Council (DHEW pub. No. (NIH) 85-23, 2985); this manuscript has been written in accordance with the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines [30].
Table 1.
Number of rats per group for all experiments in study. Rats were divided by age (3 months, 12 months) and injury type (naïve, sham or SCI).
| Experiment Group | 3 Month Rats | 12 Month Rats | ||||
|---|---|---|---|---|---|---|
| Naïve | Sham** | SCI | Naïve | Sham** | SCI | |
| PET Imaging (Naïve/1/14 dpi)* | 12 | 6 | 6 | 8 | 4 | 4 |
| qRT-PCR/Western Blot (Naïve)*** | 4 | NA | NA | 4 | NA | NA |
| qRT-PCR/Western Blot (14 dpi) | NA | 4 | 4 | NA | 4 | 4 |
| Total n | 16 | 10 | 10 | 12 | 8 | 8 |
All rats that underwent PET imaging were included in baseline quantification analysis.
For biochemistry experiments, the 1 cm section of spinal cord encompassing the lesion site at T9 was divided for qRT-PCR and western blot.
Contusion SCI
Contusion SCI was performed in rats (n=10 (3 month) and 8 (12 month)) as described previously [31]. Briefly, moderate injury (150 kDynes force) was induced with the Infinite Horizons Impactor (Precision Systems Incorporated, Natick, MA). Sham animals (n=10 (3 month) and 8 (12 month)) received laminectomy but no impact. Naïve animals (n=10 (3 month) and 8 (12 month)) received no surgery or anesthesia.
microPET/CT Imaging and Analysis
Prior to injury and at 1 and 14 dpi, PET imaging was performed to assess FDG (1.5-2 mCi injection) uptake, as previously described [17], with a 70-minute uptake period and a static 30-minute PET scan using a Siemens Inveon Multimodality scanner (Siemens Medical Solutions, Erlangen, Germany).
Images were analyzed using Siemens Inveon Research Workplace (IRW) software, version 4.2, as previously described [17]. To avoid biases introduced due to partial-volume effects, the PET data were reconstructed using iterative algorithm (ordered subset expectation maximization) and the ROI’s drawn were more than 3 times (ROI size = 26mm3) (von Leden et al., 2016) the scanner intrinsic resolution (1.4mm × 3 = 4.2mm3). Further, to restrict influence of FDG uptake in surrounding injured muscle tissue of the surgery site, ROIs were restricted to within the bony structures, as previously described [17].
Comparative RT-PCR
A 5 mm section of the spinal cord, centered at the lesion epicenter, encompassing the caudal half of the lesion site, was dissected from naïve rats or at 14 dpi and immediately frozen on dry ice and processed as previously described [14]. To determine changes in gene expression, primers were designed using the Primer-Blast tool, then obtained from Integrated DNA Technologies (IDT, Coralville, IA). Primers were designed for glucose transporters GLUT3 (sense – 5’-CCATCTCTGGTGTTCGCTGT- 3’, anti-sense – 5’- GTCTTCCAACCGCTCTTCCA- 3’), GLUT4 (sense – 5’-CAGGCCGGGACACTATACC- 3’, anti-sense – 5’-AATCACTTTCTGTGGGGCGT- 3’), and internal control (GAPDH, sense – 5’- GCTGGTCATCAACGGGAAA-3’, anti-sense – 5’-ACGCCAGTAGACTCCACGACA-3’). Reported gene expression values were determined using the ΔΔ CT method: raw CT value of each sample normalized first to CT value of the internal control (GAPDH) and then to control group (either age matched naïve or 3 month injured, dependent on analysis performed).
Western Blot
The 5 mm rostral half of the lesion site of the spinal cord was then frozen on dry ice and processed for biochemistry as previously described [14]. Antibodies against GLUT3 (20μl/ml, Abcam, RRID AB_732609) and GLUT4 (10μl/ml, Abcam, RRID AB_305554) were probed overnight at 4°C and bands were verified against the literature [32], [33]. GAPDH (0.5μl/ml; Millipore, RRID AB_2107445) was used as a control. To ensure that GAPDH was not adversely affected by age or injury, side-by-side comparison with an alternative protein loading control, β-actin, was performed and showed no difference among groups in protein expression (Supplementary Fig. 1). NIH Image J was used to assess pixel density of resultant blots for quantitation.
Statistics
Sample sizes were calculated based on our previous work [17,34]. All assays were carried out by investigators blinded to subject group. Quantitative data are presented as mean +/− standard error of the mean (SEM). Post-injury PET imaging was analyzed using a 2-way ANOVA with Tukey’s multiple comparison’s post hoc test. Baseline PET imaging, western blots and q-RT PCR were analyzed using the student t-test. All statistical tests were performed using the GraphPad Prism Program, Version 6.03 for Windows (GraphPad Software, San Diego, CA). A p value < 0.05 was considered statistically significant.
Results
Baseline FDG uptake is decreased in 12 month rat spinal cord
To determine glucose uptake in naïve 3 month and 12 month rats, FDG-PET imaging was performed and scans were analyzed using region of interest (ROI, Fig. 1A) analysis with reference region (cerebellum) normalization. 12 month rats showed a significant decrease in glucose uptake compared to 3 month rats at baseline (t(5.269), p<0.0001; Fig. 1B). To verify that these differences in glucose uptake were not region specific, we analyzed an ROI in the cervical spine using the same shape and size as at T9, which showed a similar reduction in 12 month rats (t(3.304), p=0.0064; Fig. 1B).
Figure 1.
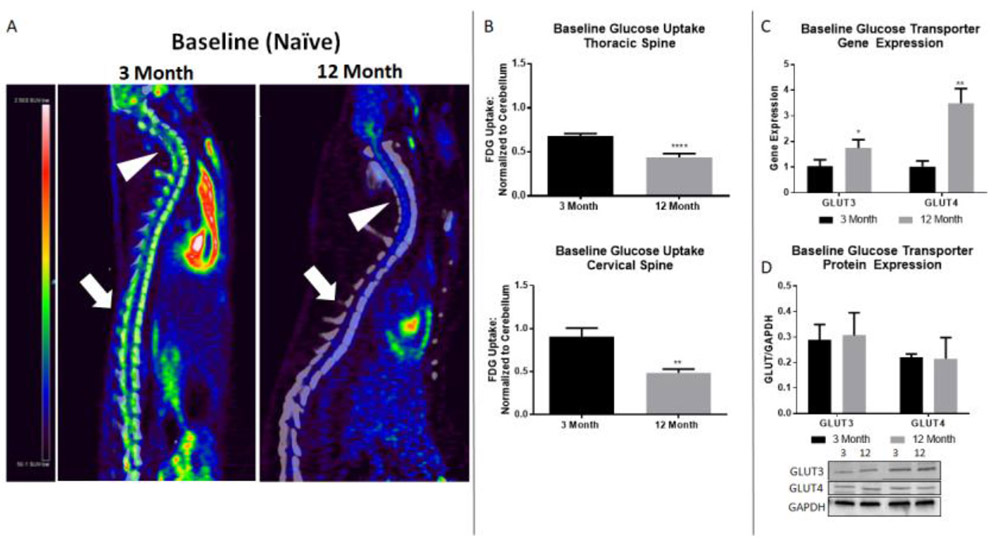
12 month rats show altered glucose uptake pattern and GLUT3/4 gene expression compared to 3 month rats. A) Representative PET images of 3 and 12 month baseline (naïve) spinal cord. Arrowheads point to cervical spine region, block arrows to thoracic injury site. Heatmap: black/blue represents lowest level of uptake, red/white represents highest level of uptake. B) Naïve 12 month rats show significant decreases in glucose uptake compared to naïve 3 month rats in both the thoracic and cervical cord. No difference in glucose uptake is found in cervical spine after injury, indicating post injury alterations in glucose uptake are specific to the injury site. C) Comparative RT-PCR between 3 month and 12 month rats reveal significantly increased gene expression in 12 month rats in both GLUT3 and GLUT4. D) No significant difference in GLUT3 or GLUT4 protein expression is found by age group. N=8-12/group (PET imaging), 4/group (gene and protein expression); *p<0.05, ** p<0.005, **** p<0.0001.
GLUT3/4 gene and protein expression is altered in 12 month rat spinal cord
To determine if age altered the glucose transport machinery, we examined gene and protein expression of GLUT3 and GLUT4 in naïve spinal cords. Interestingly, gene expression of both GLUT3 and GLUT4 was significantly increased in the 12 month rats compared to the 3 month rats (t(2.985), p=0.0405 and t(7.05), p=0.0021, respectively; Fig. 1C), showing an inverse relationship to glucose uptake. Protein levels of GLUT3 and GLUT4 were not different between age groups.
FDG uptake is increased in 12 month rats at 14 days post-injury
To determine if glucose uptake was altered by age after SCI, FDG-PET was performed at 14 dpi in 3 and 12 month rats (Fig. 2A). Analysis with a two-way ANOVA revealed a significant difference with age at injury (F(4,50)=5.69, p=0.0007). Post-hoc analysis revealed that 12 month injured rats showed significantly increased glucose uptake in comparison to 3 month injured rats (**p=0.0038; Fig. 2B). Sham-injury in the 12 month old rats resulted in a significant increase in glucose uptake compared to both 3 month sham and injured rats (#p=0.0174 and *p=0.0113, respectively; Fig. 2B). Regardless, 12 month injured rats remained the highest glucose uptake group of all age and injury groups, showing significantly increased uptake compared to both the 12 month and 3 month sham rats ($p=0.0172 and #p=0.0041, respectively; Fig. 2B). As significant differences in glucose uptake were only noted at baseline and 14 dpi, all subsequent analyses were performed at these time points.
Figure 2.
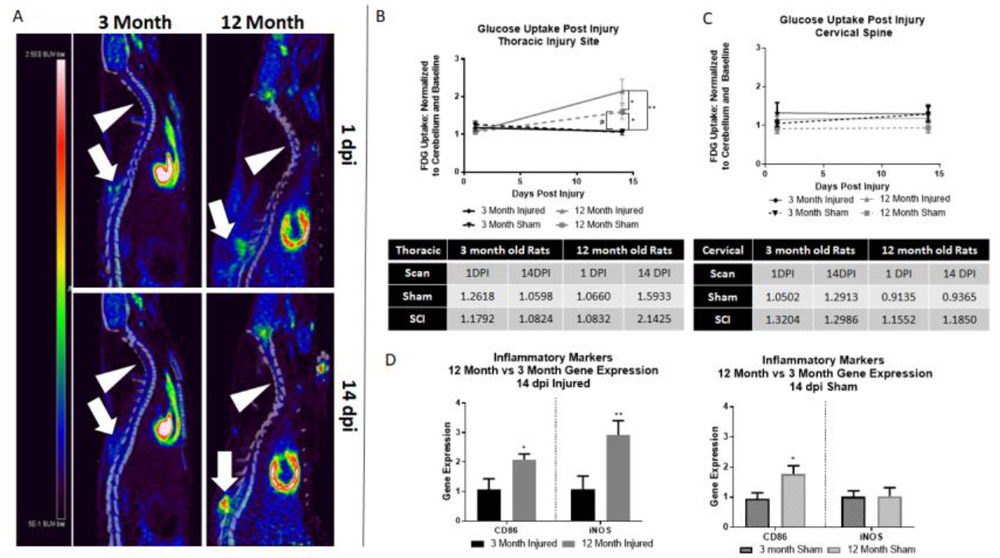
12 month rats show increased glucose uptake after SCI compared to 3 month rats. A) Representative images of both 3 and 12 month rats at 1 and 14 dpi. Arrowheads point to cervical spine, block arrows point to thoracic injury. Heatmap provided to left of images – red represents highest level of uptake. B) 12 month injured rats showed significantly higher glucose uptake than all other groups. 12 month sham rats also show significant increases in glucose uptake compared to 3 month injured (*) and sham (#) groups. Quantitative glucose uptake values in provided table (standard uptake value normalized to cerebellum control and baseline). C) At the cervical spine, no significant difference is found in any group by age or injury. D) When compared to 3 month rats, 12 month injured rats show significantly increased gene expression of both CD86 and iNOS at 14 dpi. 12 month sham rats showed significantly higher CD86 gene expression than 3 month sham rats; no significant difference was seen in iNOS by age group. N=4-6/group; *p<0.05, **p<0.005.
We examined the same size ROI in the cervical spine (C5 vertebrae) to verify that these findings were region specific and are a result of injury – two-way ANOVA analysis revealed no significant difference by age or injury in sham or injured groups (Fig. 2C).
Inflammatory marker gene expression is increased in 12 month injured rats
To determine if the inflammatory response may be related to the increased glucose uptake observed at 14 dpi in injured 12 month old rats (Fig. 2), we examined gene expression of two known pro-inflammatory markers, CD86 and iNOS. In 12 month injured rats, both CD86 and iNOS were significantly increased compared to 3 month rats (t(4.172), p=0.0140 and t(4.835), p=0.0084, respectively; Fig. 2D). Gene expression of CD86 was also significantly increased in 12 month sham rats compared to 3 month sham rats (t(3.038, p=0.0385; Fig. 2D). However, unlike 12 month injured, no difference was observed in iNOS gene expression between sham groups.
Gene and protein expression of GLUT3/4 is altered after injury
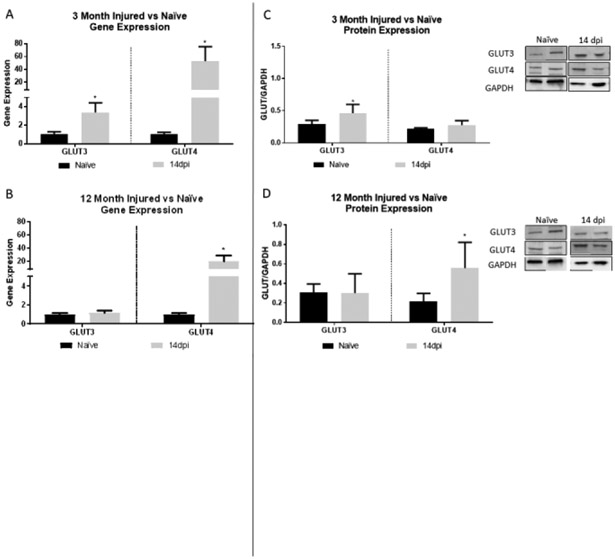
To characterize the post-injury gene and protein expression pattern of GLUT3/4 and determine if age alters that pattern, we compared all post-injury samples to the age matched naïve group as a control (Fig. 4). GLUT3 was significantly increased at 14 dpi in 3 month rats (t(3.772), p=0.0196; Fig. 4A), however 12 month rats showed no significant difference compared to naïve. At 14 dpi, GLUT4 gene expression was significantly increased in both 3 (t(3.97), p=0.0165; Fig. 4A) and 12 month rats (t(3.404), p=0.0272; Fig. 4B).
In 3 month rats, GLUT3 protein levels at 14 dpi were significantly increased compared to nai’ves (t(3.907), p=0.0298; Fig. 4C). 12 month rats also had significant increases in GLUT4 at 14 dpi (t(2.684), p=0.0500; Fig. 4D), however, they did not show any significant difference in GLUT3 protein expression.
Discussion
The results of this study show that glucose uptake is depressed at baseline in 12 month rats compared to 3 month rats, followed by a notable increase during the secondary injury period post SCI. Previous work by our group using PET imaging of glucose uptake after SCI in 3 month old rats [17] suggested an association between increased glucose uptake and inflammatory activity, and other findings by our group have shown that age results in basally increased inflammatory activity that is hyper-responsive to insult [14]. Accordingly, we now show that at 14 dpi, glucose uptake is significantly increased in 12 month old injured rats over 3 month old rats and analysis of inflammatory markers CD86 and iNOS at 14 dpi show an associated increase in inflammation chronically after injury.
Interestingly, evaluation of sham-injured middle-aged rats showed an intermediate phenotype in both glucose uptake and inflammatory response. Our previous findings [17] compared injured to sham 3 month old rats, whose glucose uptake matched naïve controls, suggesting there was no influence of the surgery alone on glucose uptake. However, in this study we found that glucose uptake in 12 month sham rats was significantly increased at 14 dpi compared to 3 month shams. As inflammatory activity in aged rats is increased in naïve tissue and hyper-responsive to insult [4,14], it is possible that laminectomy surgery alone caused an inflammatory response.
Previous work has shown that glucose metabolism is compromised and less efficient with age [19–21]. An inverse relationship between baseline glucose uptake and gene expression suggests age may influence GLUT gene expression changes in response to glucose uptake; increases in 12 month GLUT3 gene expression may be associated with age related decreases in neuronal viability, resulting in dysregulated glucose transport [35], while increased GLUT4 gene expression may indicate an attempt of the cellular environment to maintain balance in response to age-related depressed glucose uptake and increased inflammatory activity. Further, these gene expression findings may reflect a response to changes in glucose uptake, by increasing or decreasing expression levels to encourage a return to cellular homeostasis through translation of proteins. Interestingly, GLUT3/4 protein levels do not differ between age groups at baseline, suggesting age-related impairments in mRNA stability and subsequent gene degradation may contribute to inconsistent gene translation [36,37].
The thoracic dorsal contusion injury causes primarily white matter damage with less impact on the neuron-heavy grey matter regions of the spinal cord [38,39]. In the CNS, GLUT3 has been shown to be most abundant on neurons [25], and GLUT4, though present on a variety of cells including neurons, is present on inflammatory glial cells (microglia and astrocytes) that migrate to the injury site [28]. In the peripheral nervous system, GLUT4 increases in response to injury, where GLUT3 does not [40]. Similarly, in our current study, increased GLUT4 primarily in 12 month old rats observed at 14 dpi is consistent with elevated glial cells [28] and evidence of chronic inflammation with aging [4,14]. Elevated and chronic inflammation has been noted in a several studies of injury to the CNS in aged populations [5,14,41,42]. While the exact mechanism behind this elevation in inflammation is not known, oxidative stress has been suggested as one possibility [5]. Glucose metabolism dysregulation may be another potential mechanism, although additional research is needed to determine if this is a cause or an effect.
Conclusions
We now show that alterations in glucose uptake profiles and expression of GLUT 3/4 are aligned with increased inflammatory markers with injury and age. These alterations to glucose uptake, transport and metabolism could contribute to worsened injury severity, chronic inflammation, and impairments in functional recovery observed in the aging SCI population and in preclinical studies[3,5,14,43,44]. This has significant implications in therapeutic approaches for SCI; treatments that are effective in young age groups may be less effective in aged populations. Current research utilizing stem cell transplantation suggests that this therapy is equally effective in both groups, but to date only 1 therapy has been assessed in more than one age[45]. As glucose is necessary for homeostatic function of all CNS cells, our findings suggest that manipulation of glucose uptake and metabolism could be a potential therapeutic avenue for aging and/or SCI.
Supplementary Material
Figure 3.
Comparative RT-PCR and protein expression for GLUT3 and 4 in 3 and 12 month rats show alterations after SCI. A) 3 month rats show significantly increased GLUT3 and GLUT4 gene expression at 14dpi. B) 12 month rats also show significantly increased GLUT4 at 14 dpi. C) In 3 month rats, GLUT3 protein expression is significantly increased at 14dpi. D) In 12 month rats, GLUT4 protein expression is significantly increased at 14 dpi. Representative images of western blots are shown. N=4/group; *p<0.05.
Highlights.
Glucose uptake is depressed in middle-aged rats
Glucose transporter (GLUT)-3/4 show altered gene expression in middle-aged rats
Glucose uptake in increased in middle-aged rats after spinal cord injury
Inconsistencies between gene and protein suggest age-related impaired translation
Acknowledgements
The authors would like to acknowledge the contributions of Guzal Khayrullina for verification of PET imaging data and John Reed for assistance with western blot analysis.
Funding: This work was supported by a pilot grant from the Uniformed Services University. R. von Leden was supported by the NINDS/NIH (Grant number 1F31NS090737-01A1).
The opinions or assertions contained herein are the private ones of the author(s) and are not to be construed as official or reflecting the views of the DoD or the USU. The authors declare no competing financial interests.
Abbreviations:
- (DPI)
Days post-injury
- (FDG)
Fluorodeoxyglucose
- (GLUTs)
Glucose transporters
- (iNOS)
Inducible nitric oxide synthase
- (IRW)
Inveon Research Workplace
- (PET)
Positron emission tomography
- (qRT-PCR)
Quantitative Real Time PCR
- (ROI)
Region of interest (ROI)
- (SCI)
Spinal cord injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- [1].Jain NB, Ayers GD, Peterson EN, Harris MB, Morse L, O’Connor KC, Garshick E, Traumatic spinal cord injury in the United States, 1993–2012, JAMA. 313 (2015) 2236–2243. doi: 10.1001/jama.2015.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].NSCISC, National Spinal Cord Injury Statistical Center, Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham, 2016, 2016. [Google Scholar]
- [3].Hooshmand MJ, Galvan MD, Partida E, Anderson AJ, Characterization of recovery, repair, and inflammatory processes following contusion spinal cord injury in old female rats: is age a limitation?, Immun. Ageing A 11 (2014) 15. doi: 10.1186/1742-4933-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Norden DM, Muccigrosso MM, Godbout JP, Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease, Neuropharmacology. 96 (2015) 29–41. doi: 10.1016/j.neuropharm.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang B, Bailey WM, McVicar AL, Gensel JC, Age increases reactive oxygen species production in macrophages and potentiates oxidative damage after spinal cord injury, Neurobiol. Aging 47 (2016) 157–167. doi: 10.1016/j.neurobiolaging.2016.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang B, Bailey WM, Braun KJ, Gensel JC, Age decreases macrophage IL-10 expression: Implications for functional recovery and tissue repair in spinal cord injury, Exp. Neurol 273 (2015) 83–91. doi: 10.1016/j.expneurol.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ronsyn MW, Berneman ZN, Van Tendeloo VFI, Jorens PG, Ponsaerts P, Can cell therapy heal a spinal cord injury?, Spinal Cord. 46 (2008) 532–539. doi: 10.1038/sc.2008.13. [DOI] [PubMed] [Google Scholar]
- [8].Qin L, Liu Y, Hong J-S, Crews FT, NADPH oxidase and aging drive microglial activation, oxidative stress, and dopaminergic neurodegeneration following systemic LPS administration, Glia. 61 (2013) 855–868. doi: 10.1002/glia.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gwak YS, Hains BC, Johnson KM, Hulsebosch CE, Effect of Age at Time of Spinal Cord Injury on Behavioral Outcomes in Rat, J. Neurotrauma 21 (2004) 983–993. doi: 10.1089/0897715041650999. [DOI] [PubMed] [Google Scholar]
- [10].Gwak YS, Hains BC, Johnson KM, Hulsebosch CE, Locomotor recovery and mechanical hyperalgesia following spinal cord injury depend on age at time of injury in rat, Neurosci. Lett 362 (2004) 232–235. doi: 10.1016/j.neulet.2004.03.019. [DOI] [PubMed] [Google Scholar]
- [11].Siegenthaler MM, Ammon DL, Keirstead HS, Myelin pathogenesis and functional deficits following SCI are age-associated, Exp. Neurol 213 (2008) 363–371. doi: 10.1016/j.expneurol.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Morganti JM, Riparip L-K, Chou A, Liu S, Gupta N, Rosi S, Age exacerbates the CCR2/5-mediated neuroinflammatory response to traumatic brain injury, J. Neuroinflammation 13 (2016) 80. doi: 10.1186/sl2974-016-0547-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Niraula A, Sheridan JF, Godbout JP, Microglia Priming with Aging and Stress, Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 42 (2017) 318–333. doi: 10.1038/npp.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].von Leden RE, Khayrullina G, Moritz KE, Byrnes KR, Age exacerbates microglial activation, oxidative stress, inflammatory and NOX2 gene expression, and delays functional recovery in a middle-aged rodent model of spinal cord injury, J. Neuroinflammation In Press (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].McEwen BS, Reagan LP, Glucose transporter expression in the central nervous system: relationship to synaptic function, Eur. J. Pharmacol 490 (2004) 13–24. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- [16].Mergenthaler P, Lindauer U, Dienel GA, Meisel A, Sugar for the brain: the role of glucose in physiological and pathological brain function, Trends Neurosci. 36 (2013) 587–597. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].von Leden RE, Selwyn RG, Jaiswal S, Wilson CM, Khayrullina G, Byrnes KR (18)F-FDG-PET imaging of rat spinal cord demonstrates altered glucose uptake acutely after contusion injury, Neurosci. Lett 621 (2016) 126–132. doi: 10.1016/j.neulet.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ding F, Yao J, Rettberg JR, Chen S, Brinton RD, Early decline in glucose transport and metabolism precedes shift to ketogenic system in female aging and Alzheimer’s mouse brain: implication for bioenergetic intervention, PloS One. 8 (2013) e79977. doi: 10.1371/journal.pone.0079977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].dos Santos JM, Benite-Ribeiro SA, Queiroz G, Duarte JA, The effect of age on glucose uptake and GLUT1 and GLUT4 expression in rat skeletal muscle, Cell Biochem. Funct 30 (2012) 191–197. doi: 10.1002/cbf.l834. [DOI] [PubMed] [Google Scholar]
- [20].Fattoretti P, Bertoni-Freddari C, Casoli T, Di Stefano G, Solazzi M, Giorgetti B, Decreased expression of glucose transport protein (Glut3) in aging and vitamin E deficiency, Ann. N. Y. Acad. Sci 973 (2002) 293–296. [DOI] [PubMed] [Google Scholar]
- [21].Oka Y, Asano T, Lin JL, Tsukuda K, Katagiri H, Ishihara H, Inukai K, Yazaki Y, Expression of glucose transporter isoforms with aging, Gerontology. 38 Suppl 1 (1992) 3–9. [DOI] [PubMed] [Google Scholar]
- [22].Duelli R, Kuschinsky W, Brain glucose transporters: relationship to local energy demand, News Physiol. Sci. Int. J. Physiol. Prod. Jointly Int. Union Physiol. Sci. Am. Physiol. Soc 16 (2001) 71–76. [DOI] [PubMed] [Google Scholar]
- [23].Rydzewski BZ, Wozniak MM, Raizada MK, Glucose transporters in central nervous system glucose homeostasis, Adv. Exp. Med. Biol 293 (1991) 397–404. [DOI] [PubMed] [Google Scholar]
- [24].Jurcovicova J, Glucose transport in brain - effect of inflammation, Endocr. Regul 48 (2014) 35–48. [DOI] [PubMed] [Google Scholar]
- [25].Vannucci SJ, Maher F, Simpson IA, Glucose transporter proteins in brain: delivery of glucose to neurons and glia, Glia. 21 (1997) 2–21. [DOI] [PubMed] [Google Scholar]
- [26].Calder PC, Dimitriadis G, Newsholme P, Glucose metabolism in lymphoid and inflammatory cells and tissues, Curr. Opin. Clin. Nutr. Metab. Care 10 (2007) 531–540. doi: 10.1097/MCO.0b013e3281e72ad4. [DOI] [PubMed] [Google Scholar]
- [27].El Messari S, Aït-Ikhlef A, Ambroise DH, Penicaud L, Arluison M, Expression of insulin-responsive glucose transporter GLUT4 mRNA in the rat brain and spinal cord: an in situ hybridization study, J. Chem. Neuroanat 24 (2002) 225–242. [DOI] [PubMed] [Google Scholar]
- [28].Nijland PG, Michailidou I, Witte ME, Mizee MR, van der Pol SMA, van Het Hof B, Reijerkerk A, Pellerin L, van der Valk P, de Vries HE, van Horssen J, Cellular distribution of glucose and monocarboxylate transporters in human brain white matter and multiple sclerosis lesions, Glia. 62 (2014) 1125–1141. doi: 10.1002/glia.22667. [DOI] [PubMed] [Google Scholar]
- [29].Hamlin GP, Cernak I, Wixey JA, Vink R, Increased expression of neuronal glucose transporter 3 but not glial glucose transporter 1 following severe diffuse traumatic brain injury in rats, J. Neurotrauma 18 (2001) 1011–1018. doi: 10.1089/08977150152693700. [DOI] [PubMed] [Google Scholar]
- [30].Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG, Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research, PLoS Biol 8 (2010) el000412. doi: 10.1371/journal.pbio. 1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cooney SJ, Bermudez-Sabogal SL, Byrnes KR, Cellular and temporal expression of NADPH oxidase (NOX) isotypes after brain injury, J. Neuroinflammation 10 (2013) 155. doi: 10.1186/1742-2094-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kuang R, Jahangiri A, Mascharak S, Nguyen A, Chandra A, Flanigan PM, Yagnik G, Wagner JR, De Lay M, Carrera D, Castro BA, Hayes J, Sidorov M, Garcia JLI, Eriksson P, Ronen S, Phillips J, Molinaro A, Koliwad S, Aghi MK, GLUT3 upregulation promotes metabolic reprogramming associated with antiangiogenic therapy resistance, JCI Insight. 2 (2017) e88815. doi: 10.1172/jci.insight.88815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lu H, Gao Z, Zhao Z, Weng J, Ye J, Transient hypoxia reprograms differentiating adipocytes for enhanced insulin sensitivity and triglyceride accumulation, Int. J. Obes 2005. 40 (2016) 121–128. doi: 10.1038/ijo.2015.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Selwyn R, Hockenbury N, Jaiswal S, Mathur S, Armstrong RC, Byrnes KR, Mild traumatic brain injury results in depressed cerebral glucose uptake: An (18)FDGPET study, J. Neurotrauma 30 (2013) 1943–1953. doi: 10.1089/neu.2013.2928. [DOI] [PubMed] [Google Scholar]
- [35].Morrison JH, Hof PR, Life and death of neurons in the aging brain, Science. 278 (1997) 412–419. [DOI] [PubMed] [Google Scholar]
- [36].Vemace VA, Schmidt-Glenewinkel T, Figueiredo-Pereira ME, Aging and regulated protein degradation: who has the UPPer hand?, Aging Cell. 6 (2007) 599–606. doi: 10.1111/j.1474-9726.2007.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vogel C, Marcotte EM, Insights into the regulation of protein abundance from proteomic and transcriptomic analyses, Nat. Rev. Genet 13 (2012) 227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ek CJ, Habgood MD, Dennis R, Dziegielewska KM, Mallard C, Wheaton B, Saunders NR, Pathological changes in the white matter after spinal contusion injury in the rat, PloS One. 7 (2012) e43484. doi: 10.1371/journal.pone.0043484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ek CJ, Habgood MD, Callaway JK, Dennis R, Dziegielewska KM, Johansson PA, Potter A, Wheaton B, Saunders NR, Spatio-temporal progression of grey and white matter damage following contusion injury in rat spinal cord, PloS One. 5(2010) el2021. doi: 10.1371/journal.pone.0012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gómez O, Ballester-Lurbe B, Mesonero JE, Terrado J, Glucose transporters GLUT4 and GLUT8 are upregulated after facial nerve axotomy in adult mice, J. Anat 219 (2011) 525–530. doi: 10.1111/j.1469-7580.2011.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fenn AM, Hall JCE, Gensel JC, Popovich PG, Godbout JP, IL-4 signaling drives a unique arginase+/IL-1β+ microglia phenotype and recruits macrophages to the inflammatory CNS: consequences of age-related deficits in IL-4Rα after traumatic spinal cord injury, J. Neurosci. Off. J. Soc. Neurosci 34 (2014) 8904–8917. doi: 10.1523/JNEUROSCI.1146-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ, Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states, Neurobiol. Aging 34 (2013) 1397–1411. doi: 10.1016/j.neurobiolaging.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Frontera JE, Mollett P, Aging with Spinal Cord Injury: An Update, Phys. Med. Rehabil. Clin. N. Am 28 (2017) 821–828. doi: 10.1016/j.pmr.2017.06.013. [DOI] [PubMed] [Google Scholar]
- [44].Roozbehi A, Joghataei MT, Bakhtiyari M, Mohammadi J, Rad P, Delaviz H, Age-associated changes on axonal regeneration and functional outcome after spinal cord injury in rats, Acta Med. Iran 53 (2015) 281–286. [PubMed] [Google Scholar]
- [45].Takano M, Kawabata S, Shibata S, Yasuda A, Nori S, Tsuji O, Nagoshi N, Iwanami A, Ebise H, Horiuchi K, Okano H, Nakamura M, Enhanced Functional Recovery from Spinal Cord Injury in Aged Mice after Stem Cell Transplantation through HGF Induction, Stem Cell Rep 8 (2017) 509–518. doi: 10.1016/j.stemcr.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.