Figure 3:
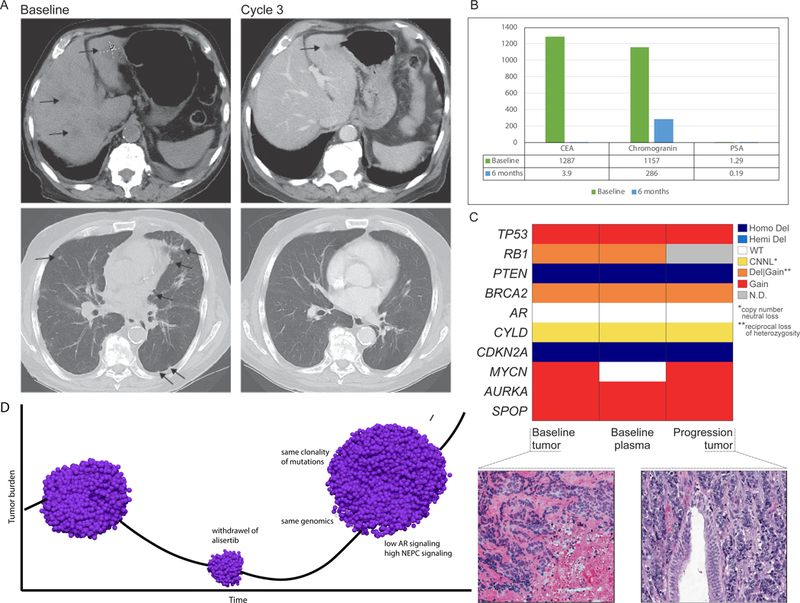
Exceptional responder patient 155 (A) CT scan at baseline (pre-treatment) and after cycle 3; (B) Serum chromogranin, CEA, PSA at baseline and after six months on alisertib; (C) Comparison of copy number of representative genes (determined by WES) showing concordance between baseline biopsy, baseline ctDNA, and progression biopsy; colors represent allele specific copy number states; hematoxylin and eosin (H&E) images showing pre-treatment baseline tumor biopsy and post-treated (progression) tumor both showing similar morphology (small cell carcinoma); (D) Schematic of patient 155 tumor response and progression after therapeutic release from alisertib (drug was held 17 days). pre-treatment biopsy: AR signaling score 0.04; NEPC score 0.5; progression tumor: AR signaling score 0.05, NEPC score 0.47.
