Abstract
Background:
Heart failure (HF) is a risk factor for atrial fibrillation (AF), stroke, and post-stroke disability. However, differing definitions and application of HF-criteria may impact model prediction. We compared the predictive ability of left ventricular ejection fraction (LVEF), a readily available objective echocardiographic index, with clinical HF definitions for functional disability and AF in stroke patients.
Methods:
We retrospectively analyzed ischemic stroke patients evaluated between January 2013 and May 2015. Outcomes of interest were: (a) 90-day functional disability (modified Ranking Score 3-6) and (b) AF. We compared: (1) LVEF (continuous variable), (2) left ventricular systolic dysfunction (LVSD)-categories (absent to severe), (3) clinical history of HF, and (4) HF/LVSD-categories: (i) HF absent without LVSD, (ii) HF absent with LVSD, (iii) HF with preserved ejection fraction (HFpEF), and (iv) HF with reduced ejection fraction (HFrEF). Multivariable logistic regression was used to determine the predictive ability for 90-day disability and AF, respectively.
Results:
685 consecutive patients (44.5% female) fulfilled the study criteria and were included. After adjustment, the LVEF was independently associated with 90-day disability (OR 0.98, 95%-CI 0.96-0.99, P=0.011) with similar predictive ability (area under the curve [AUC] 0.85) to models including the LVSD-categories (AUC 0.85), clinically define HF (AUC 0.86), and HF/LVSD-categories (AUC 0.86). The LVEF, HF, LVSD-, and HF/LVSD-categories were independently associated with AF (P<0.01, each) with similar predictive ability (AUC 0.74, 0.74, 0.73, and 0.75, respectively).
Conclusions:
Compared to commonly defined HF definitions, the objectively determined LVEF possesses comparable predictive ability for 90-day disability and AF in stroke patients.
Keywords: atrial fibrillation, cardioembolism, disability, heart failure, HFpEF, HFrEF, outcome, TOAST
Introduction
Heart failure (HF) is a recognized risk factor for near and long-term risk of stroke that may be useful to predict post stroke functional disability, death and atrial fibrillation given the shared underlying risk factors.1-5 However, the definition and physician application of HF criteria have been shown to vary substantially.6-8 For example, the functional classification based on the widely-used New York Heart Association (NYHA) definition relies on subjective and self-reported criteria. Although this classification has been validated,9 it has nevertheless been shown to suffer suboptimal reproducibility and lack of sensitivity.10,11 This increases the risk for bias, particularly in studies that rely on medical record review or when information is difficult to extract such as in subjects with aphasia or cognitive impairment.
Accordingly, it may be advantageous to define the severity of HF based on objective indices such as the echocardiographically defined left ventricular ejection fraction (LVEF) when investigating the association of HF and stroke. Indeed, the severity of left ventricular systolic dysfunction (LVSD), as determined by a reduced LVEF, has been found to inversely correlate with the 3-month functional outcome after stroke.2 Furthermore, several lines of data suggest that a reduced LVEF represents the greatest stroke risk factor when multiple definitions of HF are used.8 This is an important observation because an increasing proportion of HF patients are diagnosed with HF and preserved left ventricular ejection fraction (HFpEF).12 Yet, there is a striking paucity of contemporary studies that systematically explored the potential relation of different HF definitions with post-stroke disability.2,4,13,14 Understanding this issue is important because there is increasing interest to use established stroke scales that incorporate HF (such as the CHA2DS2-VASc score) to stratify stroke risk and outcome as well as AF.15-18
To elucidate this issue, we sought to determine whether the use of different HF definitions impact outcome prediction and AF in ischemic stroke. Given the complexity of defining HF based on clinical grounds we used the echocardographically defined LVEF both as continuous variable as well as stratified according to LVSD-category. We hypothesized that the LVEF may serve as objective HF-index to predict the 90-day functional outcome as well as AF among patients with ischemic stroke as compared to commonly used clinical HF definitions.
Materials and Methods
Study cohort
We retrospectively analyzed 685 patients with acute ischemic stroke that were consecutively included in the University of Massachusetts Memorial Medical Center Stroke registry between January 2013 and May 2015.
Our investigation was approved by our Institutional Review Board (#H00006964) and Health Insurance Portability and Accountability Act waiver of informed consent granted. We adhere to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines (www.strobe-statement.org).
Clinical characteristics, stroke etiology, and stroke severity
At baseline, all study participants underwent a standardized clinical history, physical examination, head CT or 1.5 T brain MRI (typically obtained within 24-48 hours after the symptoms onset) per institutional protocol. Patient demographics, laboratory data, co-morbidities, and pre-admission medications were abstracted from the medical record by trained physician abstractors. Members of the stroke team certified in NIHSS scoring graded the severity of stroke at presentation. The stroke etiology was determined based on the Trial of ORG-10172 in Acute Stroke Treatment (TOAST) classification after completion of the stroke workup.19 In addition, we defined embolic stroke of undetermined source (ESUS) according to the criteria proposed by the Cryptogenic Stroke/ESUS International Working Group as a visualized no-lacunar brain infarct in the absence of the following: (1) extracranial or intracranial atherosclerosis causing ≥50% luminal stenosis in arteries supplying the area of ischemia, (2) major-risk cardioembolic source, and (3) any other specific cause of stroke (e.g., arterial dissection).20 We trichotomized the stroke cause according the presumed mechanism to cardioembolic stroke (CES), non-embolic stroke (Non-ES; i.e., large vessel disease, small vessel disease, and other determined etiology), and ESUS for statistical purposes.
Risk factor definitions
We determined the presence of hypertension (use of antihypertensive medications, or systolic blood pressure of ≥140 mm Hg or diastolic blood pressure of ≥90 mm Hg on 2 separate occasions), hypercholesterolemia (use of lipid-lowering agents, or fasting blood total cholesterol level of ≥200 mg/dl, or low density lipoprotein cholesterol [LDLc] of ≥130 mg/dL), and diabetes mellitus (history of fasting glucose ≥126 mg/dL or current use of hypoglycemic drugs) as defined according to the National Diabetes Data Group and World Health Organization.21
Clinically defined heart failure and left ventricular dysfunction
All patients underwent cardiac workup including a transthoracic echocardiogram (TTE) or transesophageal echocardiogram (TEE) with evaluation of the LVEF. For the purpose of this study we used the following definitions of heart failure:
First, we clinically defined HF based on the patients’ subjective report or as documented in the incident admission records as well as primary care physician and cardiologist notes (“clinical HF”). Of note, the NYHA classification was not used because the required information to define the specific categories was not reliably available in the medical records.
Second, we determined the LVEF from 2D images using Simpson’s biplane method of discs as previously described22 and as adjudicated by a board-certified cardiologist (T.P.F.) masked to clinical data and study outcomes to avoid biased interpretation. In addition to using the LVEF as continuous variable (“LVEF”) in analyses we also stratified patients according to the degree of LVSD (“LVSD categories”) to (i) absent (LVEF of 53-73%), (ii) mild LVSD (LVEF 41-52%), (iii) moderate (LVEF 30-40%), or (iv) severe LVSD (LVEF <30%).2,22,23
Third, we stratified subjects based on the presence of clinical HF (absent versus present) and LVSD (i.e., presence versus absence of a reduced LVEF of <53%) to the following “HF/EF” categories: (i) clinical HF absent without LVSD, (ii) clinical HF absent with LVSD, (iii) clinical HF present with LVSD (i.e., HF with preserved ejection fraction; HFpEF), and (iv) clinical HF present with LVSD (i.e., HF with reduced ejection fraction; HFrEF).6,24
Atrial fibrillation
All participants were screened for incident AF with 12-lead electrocardiogram (ECG) at presentation and 24-hour in-patient cardiac telemetry with automated arrhythmia detection per institutional protocol. Additional long-term rhythm monitoring was done at the treating physician’s discretion. AF was defined according to the American Heart Association Atrial Fibrillation Management Guidelines.25 Patients with AF in the setting of rheumatic mitral valve disease, a prosthetic heart valve, or mitral valve repair (valvular AF) were included in this study.25,26 Given our study design it was no possible to reliably determine the AF duration and AF burden. Thus, for the purposes of our analyses, we considered AF present if AF of any duration was documented in the admission history, or if present on an admission 12-lead ECG. In addition, abstractors reviewed all clinical notes, 30-day non-invasive monitor reports, implantable device reports (including pacemaker and implantable loop recorders), as well as all 12-lead ECGs obtained for any reason for newly diagnosed AF of any duration during hospitalization and within 90 days of index hospital discharge.
The CHA2DS2-VASc score (range 0 to 9) was calculated based on data available in the medical record (HF, hypertension, age ≥75 years (doubled), diabetes mellitus, prior stroke/TIA (doubled), vascular disease, age 65-74 years, sex category [female]). The qualifying stroke/TIA was counted towards the CHA2DS2-VASc-score; hence the minimal possible score in our cohort was 2.
Functional outcome
The modified Rankin Scale (mRS) was assessed at discharge and 90 days after admission by a stroke-trained physician or stroke study nurse certified in mRS via in-person or telephone interview. When the mRS was unavailable, the same observers reconstructed the score from the case description according to the mRS criteria. The 90-day outcome was dichotomized to good (mRS score 0–2; disability free) versus poor (mRS score 3–6; disabled).
Statistics
Unless otherwise stated, continuous variables are reported as mean ± S.D. or median (25th-75th percentile). Categorical variables are reported as proportions. Between-group comparisons for continuous and ordinal variables were made with Mann-Whitney U test and Kruskal–Wallis one-way ANOVA on ranks with post hoc Dunn’s test as appropriate. Categorical variables were compared using the χ2-test. Bonferroni method was used to correct for multiple comparisons.
We created separate multivariable logistic regression models to determine whether (i) clinical HF, (ii) HF/EF, (iii) LVEF, and (iv) LVSD-categories predicted 90-day disability (dependent variable, primary analyses). Models were adjusted for the stroke severity (as assessed by the admission NIHSS), treatment with rtPA, and the trichotomized stroke cause. In addition, we adjusted all models for risk factors included in the CHA2DS2-VASc risk score, which is increasingly used to predict stroke and atrial fibrillation risk as well as post-stroke outcome.3,15,17 For secondary analyses we created separate multivariable logistic regression models to determine whether the (i) clinical HF, (ii) HF/EF, (iii) LVEF, and (iv) LVSD-categories predicted the presence of AF (dependent variable). These models were adjusted for risk factors included in the CHA2DS2-VASc risk score.
To obtain better comparability of odds ratio (OR) strengths with other studies based on the CHA2DS2-VASc we transformed metric measures into the same categorical variables according to CHA2DS2-VASc score determination. To avoid model overfitting, variables were sequentially removed (likelihood ratio) from the models at a significance level of 0.1. Collinearity diagnostics were performed (and its presence rejected) for all multivariable regression models. The Hosmer-Lemeshow goodness-of-fit statistic was used to assess model fit. To determine the diagnostic accuracy of the different HF definitions for functional outcome and AF, we calculated the area under receiver-operator curves (AUC; c-statistics) with ponding 95% CIs. Two-sided significance tests were used throughout and unless stated otherwise a two-sided P<0.05 was considered statistically significant. All statistical analyses were performed using IBM® SPSS® Statistics Version 22 (IBM®-Armonk, NY).
Results
Study cohort
Over the study period a total of 827 adult ischemic stroke patients were evaluated by our stroke team. After independent chart review we excluded 24 (0.3%) patients as they had no imaging confirmed stroke or because they represented duplicate entries. 118 (14.2%) subjects had no TTE or TEE available for review, leaving 685 patients for analysis (Figure 1). Data was complete for all variables except for the 90-day mRS because 69 (10.1%) subjects were lost to follow up. For these subjects, we imputed the 90-day mRS by carrying forward the discharge mRS.
Figure 1. Flow Chart.
Compared to included patients, excluded patients were older (P=0.001), had a higher admission NIHSS (P=0.002), and had more often goals of care shifted to comfort measures only (CMO; P<0.001). However, there was no difference in sex (P=0.27) and history of HF (P=0.53).
Overall, 240 (35.0%) of the studied patients had a poor 90-day functional outcome. On average, these patients were older (76 vs. 67 years, P<0.001), more frequently female (54.6% vs. 39.1%, P<0.001), had worse neurological deficits at admission (NIHSS 13 vs. 3, P<0.001), and had more vascular risk factors, when compared to patients with a good functional outcome (Table 1). The stroke mechanism was CES in 151 (22.0%), ESUS in 128 (18.7%), and non-ES in 406 (59.3%) patients. Non-ES causes related to large artery atherosclerosis in 175 (25.5%), small vessel disease in 85 (12.4%), multiple coexisting causes in 96 (14.0%), and other determined causes in 50 (7.3%) subjects.
Table 1.
Baseline characteristics (unadjusted) of the studied patient population as stratified by the 90-day outcome
| All patients | Good outcome | Poor outcome | |||
|---|---|---|---|---|---|
| Characteristics | (n=685) | (n=445) | (n=240) | P-value | |
| Age, years | 70 (60-80) | 67(59-78) | 76 (63-86) | <0.001 | |
| Female sex | 305 (44.5) | 174 (39.1) | 131 (54.6) | <0.001 | |
| Admission NIHSS | 5 (2-12) | 3 (1-7) | 13 (6-19) | <0.001 | |
| Stroke mechanism | <0.001 | ||||
| Non-embolic* | 406 (59.3) | 273 (61.3) | 133 (55.4) | ||
| Cardioembolic | 151 (22.0) | 75 (16.9) | 76 (31.7) | ||
| ESUS | 128 (18.7) | 97 (21.8) | 31 (12.9) | ||
| Preexisting risk factors | |||||
| Hypertension | 535 (78.1) | 334 (75.1) | 201 (83.8) | 0.009 | |
| Diabetes | 195 (28.5) | 113 (25.4) | 82 (34.2) | 0.017 | |
| Prior stroke or TIA | 161 (23.5) | 92 (20.7) | 69 (28.8) | 0.018 | |
| Peripheral artery disease | 199 (29.1) | 117 (26.3) | 82 (34.2) | 0.034 | |
| Hyperlipidemia | 378 (55.2) | 246 (55.3) | 132 (55.0) | 1.000 | |
| Atrial fibrillation | 204 (29.8) | 108 (24.3) | 96 (40.0) | <0.001 | |
| CHA2DS2-VASc score | 5 (4-6) | 4 (3-5.5) | 6 (4-6) | <0.001 | |
| Left ventricular ejection fraction (LVEF), % | 65 (65-65) | 62 (65-65) | 60 (65-65) | 0.004 | |
| LVSD-categories ** | 0.023 | ||||
| Absent LVSD (LVEF >52) | 599 (87.4) | 401 (90.1) | 198 (82.5) | ||
| Mild LVSD (LVEF 41-52) | 31 (4.5) | 18 (4.0) | 13 (5.4) | ||
| Moderate LVSD (LVEF 30-40) | 34 (5.0) | 15 (3.4) | 19 (7.9) | ||
| Severe LVSD (LVEF <30) | 21 (3.1) | 11 (2.5) | 10 (4.2) | ||
| HF/EF-categories** | <0.001 | ||||
| Clinical HF absent without LVSD | 553 (80.7) | 382 (85.8) | 171 (71.3) | ||
| Clinical HF absent with LVSD | 52 (7.6) | 28 (6.3) | 24 (10.0) | ||
| Clinical HF present with preserved LVEF | 46 (6.7) | 19 (4.3) | 27 (11.3) | ||
| Clinical HF present with reduced LVEF | 34 (5.0) | 16 (3.6) | 18 (7.5) | ||
| Clinical HF | 80 (11.7%) | 35 (7.9) | 45 (18.8) | <0.001 | |
| Preadmission medications | |||||
| Statin | 326 (47.6) | 210 (47.2) | 116 (48.3) | 0.810 | |
| Antihypertensive | 476 (69.5) | 290 (65.2) | 186 (77.5) | 0.001 | |
| Antiglycemic | 140 (20.4) | 83 (18.7) | 57 (23.8) | 0.136 | |
| Antiplatelets | 359 (52.4) | 216 (48.5) | 143 (59.6) | 0.006 | |
| Oral anticoagulants | 65 (9.5) | 33 (7.4) | 32 (13.3) | 0.014 | |
| Thrombolysis with rtPA | 141 (20.6) | 85 (19.1) | 56 (23.3) | 0.199 |
Data are n (%) or median (25th-75th quartile); ESUS= Embolic stroke of undetermined source, TIA=transient ischemic attack, NIHSS=National Institutes of Health Stroke Scale, rtPA=recombinant tissue-type plasminogen activator.
includes large artery atherosclerosis, small vessel disease, other determined, and undetermined [not ESUS]).
LVEF of <53% denoted a reduced LVEF and the presence of LVSD.
With respect to cardiac workup for AF, 98.7% patients in the entire cohort underwent ECG at admission in addition to routine 24-hour in-patient cardiac telemetry. 234 (34.2%) patients underwent 30-day event monitoring, and 52 (7.6%) patients had implantable loop recorder or ICD data available for review. Among ESUS patients, 98.4% had an admission ECG, 10.2% underwent TEE in addition to TTE, and 76.6% underwent long-term rhythm monitoring.
Based on the used HF definitions, 80 (11.7%) subjects were diagnosed with clinical HF, 86 (12.6%) had LVSD, and 132 (19.3%) were deemed to have either HFpEF (n=46), HFrEF (n=34), or absent HF with reduced LVEF (n=52) (Table 1). The association of clinical variables with the HF/EF categories and LVSD categories is summarized in Table 2 and Supplemental Table 1, respectively. Notably, while HFpEF subjects had a (by definition) significantly lower LVEF than subjects with HFrEF (P<0.05), there was no significant difference between these categories for any other clinical variable as well as the 90-day functional outcome after adjustment for multiple comparisons (P>0.05, each).
Table 2.
Baseline characteristics of the studied patient population as stratified by HF/EF categories.
| Absent clinical HF |
Absent clinical HF |
Clinical HF with | Clinical HF with |
||
|---|---|---|---|---|---|
| without LVSD | with LVSD | preserved LVEF (HFpEF) |
reduced LVEF (HFrEF) |
||
| Characteristics | (n=553) | (n=52) | (n=46) | (n=34) | P-value |
| Age, years | 68 (59-79) | 73 (65-82) | 80 (71-86) | 75 (64-88) | <0.001 |
| Female sex | 251 (45.4) | 18 (34.6) | 26 (56.5) | 10 (29.4) | 0.045 |
| Admission NIHSS | 5 (2-11) | 5 (2-13) | 9 (3-21) | 6 (3-12) | 0.034 |
| Stroke mechanism | <0.001 | ||||
| Non-embolic* | 350 (63.3) | 22 (42.3) | 21 (45.7) | 13 (38.2) | |
| Cardioembolic | 98 (17.7) | 20 (38.5) | 20 (43.5) | 13 (38.2) | |
| ESUS | 105 (19.0) | 10 (19.2) | 5 (10.9) | 8 (23.5) | |
| Preexisting risk factors | |||||
| Hypertension | 419 (75.8) | 44 (84.6) | 42 (91.3) | 30 (88.2) | 0.019 |
| Diabetes | 150 (27.1) | 19 (36.5) | 12 (26.1) | 14 (41.2) | 0.170 |
| Prior stroke or TIA | 124 (22.4) | 13 (25.0) | 11 (23.9) | 13 (38.2) | 0.217 |
| Peripheral artery disease | 115 (20.8) | 25 (48.1) | 31 (67.4) | 28 (82.4) | <0.001 |
| Hyperlipidemia | 292 (52.8) | 31 (59.6) | 30 (65.2) | 25 (73.5) | 0.042 |
| Atrial fibrillation | 138 (25.0) | 22 (42.3) | 27 (58.7) | 17 (50.0) | <0.001 |
| CHA2DS2-VASc score | 5 (3-6) | 6 (5-7) | 6 (5-6) | 6 (5-7) | <0.001 |
| LVEF, % | 65 (65-65) | 40 (35-45) | 65 (65-65) | 30 (25-41) | <0.001 |
| Preadmission medications | |||||
| Statin | 244 (44.1) | 29 (55.8) | 31 (67.4) | 22 (64.7) | 0.002 |
| Antihypertensive | 369 (66.7) | 34 (65.4) | 42 (91.3) | 31 (91.2) | <0.001 |
| Antiglycemic | 113 (20.4) | 9 (17.3) | 10 (21.7) | 8 (23.5) | 0.891 |
| Antiplatelets | 279 (50.5) | 26 (50.0) | 27 (58.7) | 27 (79.4) | 0.007 |
| Oral anticoagulants | 38 (6.9) | 9 (17.3) | 12 (26.1) | 6 (17.6) | <0.001 |
| Thrombolysis with rtPA | 107 (19.3) | 7 (13.5) | 15 (32.6) | 12 (35.3) | 0.016 |
| Good 90-day outcome | 382 (69.1) | 28 (53.2) | 19 (41.3) | 16 (47.1) | <0.001 |
Data are n (%) or median (25th-75th quartile); ESUS= Embolic stroke of undetermined source, HF=heart failure, LVEF=left ventricular ejection fraction, LVSD=left ventricular dysfunction, TIA=transient ischemic attack, NIHSS=National Institutes of Health Stroke Scale, rtPA=recombinant tissue-type plasminogen activator.
includes large artery atherosclerosis, small vessel disease, other determined, and undetermined [not ESUS]).
151 (22%) patients had a known history of AF and an additional 53 (7.7%) were newly diagnosed with AF either during admission (n=23 [3.4%]) or during the 90 days of follow up (n=30 [4.4%]). On average, patients with a poor outcome more frequently had AF than patients with a good outcome (40% vs. 24.3%, p<0.001). Among patients without a known history of AF, there was no significant difference in the proportion of newly diagnosed AF between patients with a good (39/376 [10.7%]) vs. poor outcome (14/158 [8.9%]; p=0.638).
Association of HF with the 90-day functional outcome
In unadjusted analysis, clinical HF, HF/EF, LVEF, and LVSD-categories were associated with poor stroke 90-day outcome, respectively (p<0.05 each, Tables 1-3 and Supplemental Table 1). After adjustment, for variables included in the CHA2DS2-VAsc, AF, stroke mechanism, and stroke severity all HF definitions were independently associated with 90-day disability (Table 3). Examination of the c-statistics (AUC) indicated that there was no significant difference in the predictive ability of the models using different definitions for the 90-day functional outcome (Figure 2A).
Table 3.
Multivariable logistic regression analysis for factors independently associated with a poor 90-day outcome.
| Independent variable |
Crude OR (95% CI) |
P-value | Adjusted OR (95%-CI) |
P-value | |
| Cardioembolic stroke cause | Reference | Reference | |||
| Non-embolic stroke cause | 0.48 (0.33-0.70) | <0.001 | 0.76 (0.4.6-1.26) | 0.290 | |
| ESUS | 0.32 (0.19-0.53) | <0.001 | 0.41 (0.21-0.80) | 0.008 | |
| LVEF* | 0.98 (0.96-0.99) | 0.004 | 0.98 (0.96-0.99) | 0.011 | |
| Hypertension | 1.713 (1.143- 2.567) |
0.009 | -- | -- | |
| Age ≤64 years | Reference | Reference | |||
| Age 65-74 years | 0.88 (1.04-1.60) | 0.876 | 0.90 (0.53-1.55) | 0.709 | |
| Age ≥ 75 years | 2.63 (1.82-3.81) | <0.001 | 2.11 (1.30-3.44) | 0.003 | |
| Diabetes mellitus | 1.53 (1.08-2.15) | 0.016 | 2.11 (1.37-3.26) | 0.001 | |
| Prior stroke/TIA | 1.55 (1.08-2.22) | 0.018 | 1.79 (1.14-2.81) | 0.012 | |
| Vascular disease | 1.46 (1.04-2.04) | 0.031 | 1.56 (1.21-2.02) | 0.001 | |
| Female sex | 1.87 (1.36-2.57) | <0.001 | 1.52 (1.01-2.30) | 0.047 | |
| Atrial fibrillation | 2.08 (1.49-2.91) | <0.001 | -- | -- | |
| Admission NIHSS | 1.20 (1.16-1.23) | <0.001 | 1.23 (1.18-1.27) | <0.001 | |
| iv rtPA treatment | 1.29 (0.88-1.89) | 0.192 | 0.50 (0.30-0.84) | 0.008 |
Numbers of patients in each category are shown in table 1. Odds ratios indicate the increased or decreased odds of the clinical factor being present, for a 1 percent increase in the left ventricular ejection fraction (LVEF), or a 1 point increase in the admission National Institutes of Health Stroke Scale (NIHSS) score. We used p≤0.05 as criteria for backward steps. Blank cells represent variables that were dropped as non-significant during stepwise selection. EF and NIHSS score were entered into the model as continuous variables; thus the odds ratio for ejection fraction represents the estimated change in odds of the outcome for a 1 percent increase in the ejection fraction, with all other variables unchanged. The units for NIHSS score are points on a scale from 0 to 40 (maximum observed in this study). Hosmer-Lemeshow goodness of fit χ2 13.887, P=0.085. ESUS indicates embolic stroke of undetermined source; iv rtPA, intravenous recombinant tissue plasminogen activator; TIA, transient ischemic attack.
Note that entering the clinically defined heart failure, left ventricular systolic dysfunction, and heart failure with or without reduced ejection fraction instead of the LVEF did not meaningfully change the results and are thus not shown. Also, when age was entered as continuous variable, the association of the LVEF with the 90-day outcome was not substantially affected (OR 0.98, 95%-CI 0.96 to 0.99, P=0.011).
Figure 2. Area under the receiver operating characteristic curves (AUC) for outcome and atrial fibrillation (AF) prediction.
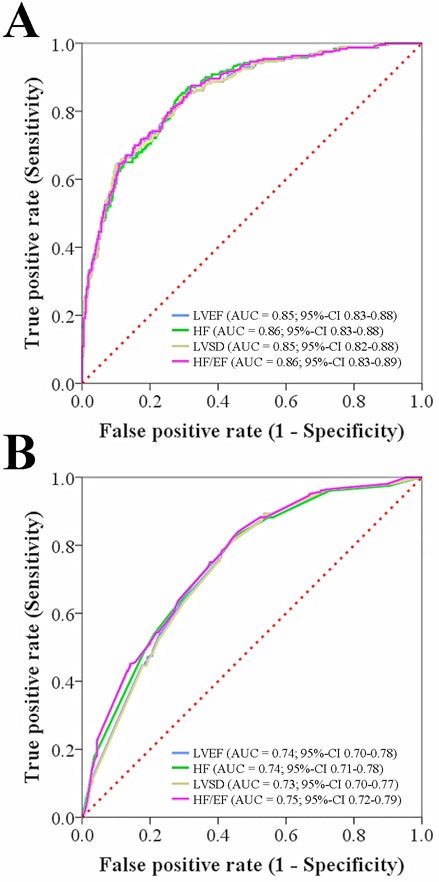
Multivariable models using the LVEF and different HF definitions showed similar predictive ability for the (A) 90-day functional outcome and (B) diagnosis of AF in the entire cohort. LVEF, indicates left ventricular ejection fraction, HF=clinical heart failure, LVSD=left ventricular dysfunction (defined as reduced LVEF of <53%), HF/EF=clinical heart failure with or without reduced ejection fraction.
Association of HF with AF
Patients with AF had a significantly lower LVEF than subjects without AF (59.5±12.0 versus 62.1±9.0, P=0.002). In unadjusted analyses, AF was significantly more common among subjects with clinical HF than those without HF (55.0% vs. 26.4%, P<0.001). AF was significantly less common in subjects without LVSD as compared to patients with LVSD (P=0.009, Supplemental Table 1) without significant difference between mild, moderate, and severe LVSD after adjustment for multiple comparisons (P>0.05, each). Lastly, AF was significantly less common in subjects with no HF and without LVSD as compared to all other HF/EF categories (P<0.001, Table 2) without difference between absent HF with LVSD, HFpEF, and HFrEF after adjustment for multiple comparisons (P>0.05, each).
The respective associations of clinical HF, HF/EF, LVEF, and LVSD-categories with AF remained after adjustment for pertinent covariates (P<0.05 each; Table 4). Examination of the c-statistics (AUC) indicated that there was no significant difference in the predictive ability of the models using the different definitions for the presence of AF, respectively (Figure 2B).
Table 4.
Multivariable logistic regression analysis for factors independently associated with atrial fibrillation in the entire cohort.
| Independent variable |
Crude OR (95% CI) |
P-value | Adjusted OR (95%-CI) |
P-value | |
|---|---|---|---|---|---|
| LVEF* | 0.98 (0.96-0.99) | 0.003 | 0.98 (0.96-0.99) | 0.009 | |
| Hypertension | 2.51 (1.58-4.00) | <0.001 | 1.73 (1.04-2.87) | 0.036 | |
| Age ≤64 years | Reference | Reference | |||
| Age 65-74 years | 3.55 (2.13-5.90) | <0.001 | 3.47 (2.06-5.85) | <0.001 | |
| Age ≥ 75 years | 7.27 (4.58-11.54) | <0.001 | 6.54 (2.06-10.54) | <0.001 | |
| Diabetes mellitus | 0.70 (0.48-1.02) | 0.063 | 0.54 (0.36-0.81) | 0.001 | |
| Prior stroke/TIA | 1.17 (0.80-1.71) | 0.425 | -- | -- | |
| Vascular disease | 1.29 (0.91-1.84) | 0.155 | -- | -- | |
| Female sex | 1.30 (0.93-1.80) | 0.124 | -- | -- |
Table 3. Hosmer-Lemeshow goodness of fit χ2 1.163, P=0.979. TIA indicates transient ischemic attack.
Note that entering the clinically defined heart failure, left ventricular systolic dysfunction, and heart failure with or without reduced ejection fraction instead of the LVEF did not meaningfully change the results and are thus not shown. Also, when age was entered as continuous variable, the association of the LVEF with atrial fibrillation was not substantially affected (P=0.008).
Discussion
HF has been associated with both a worse outcome after ischemic stroke as well as AF.2-4,27 Because commonly used clinical definitions of HF may suffer suboptimal reproducibility and lack of sensitivity, particularly in retrospective studies, we sought to determine whether the echocardiographically defined LVEF may serve as objective HF-index to predict functional disability after ischemic stroke. The most important finding of our study was that the predictive ability of the LVEF for 90-day disability as well as AF was similar to all clinically defined HF definitions.
This finding suggests equipoise for using the LVEF and frequently used clinical HF-definitions to predict outcome, results that may facilitate research as it provides the rationale for researchers to choose a HF definition that they can most reliably obtain. In this respect the LVEF is of particular interest because it is a readily available and easy to interpret echocardiographic metric that does not require detailed clinical information for diagnosing HF.
Several prior studies investigated the potential association between HF and the LVEF with post-stroke functional outcome;13,28-31 however, none directly compared the possible impact of different HF definition for predicting outcome, which impairs comparison of results across studies. In this respect it is noteworthy that the prevalence of HF in our cohort is consistent with prior studies indicating generalizability of our results.29,30,32,33 Depending of the definition used in our study the prevalence of HF ranged from 11.7% to 19.3%—a difference driven by the classification of subjects to have asymptomatic LVSD (i.e., absent HF with reduced LVSD). This is a striking observation as it suggests that asymptomatic LVSD, which was graded as moderate-to-severe LVSD in our cohort and represents a precursor for symptomatic HF,24 is an equally strong predictor for poor functional status after ischemic stroke as HFpEF and HFrEF.33
Patients with HF are at high risk for developing AF, which frequently complicates HF, affecting approximately one third of all adults with HF,4 as well as presents a major risk factor for subsequent ischemic stroke.1 Accordingly, it is important to understand the association between HF and AF in stroke patients. However, relatively few contemporaneous studies have sought to determine the impact of different HF definition on AF prediction.5 Similar to the 90-day functional outcome we found equipoise for the models incorporating the LVEF and the different used HF definitions for predicting AF in our cohort. These results are not dissimilar to data reported from a larger population based study in approximately 24,000 adults with a history of HF that found similar odds ratios HFpEF and HFrEF for predicting AF. Hence, our data indicates that the LVEF may serve as a predictor for AF in stroke patients and may serve as the impetus for conducting further studies to confirm this hypothesis. It would be particularly interesting to assess whether the LVEF and clinically defined HF can be used to predict AF in patients with ESUS (i.e., subjects with presumed, but unknown, AF diagnosis)—and issue that we could not address in our study due to the overall low number of subjects with both new onset AF and HF (<5%) rendering analyses underpowered.
Strengths of our study relate to the investigation of a well-characterized patient population with collection of pertinent clinical variables, definition of HF based on several frequently used schemes, as well as rigorous adjustment of all analyses. This study is subject to the limitations inherent to its retrospective design. With respect to our exploratory analyses, not all ESUS patient underwent long-term rhythm monitoring for covert AF, the approach to rhythm monitoring after discharge was at the discretion of the treating physician, and the overall number of included subjects was modest. Accordingly, new onset AF may have been missed in a subset of patients and further study in a larger ESUS cohort with standardized rhythm monitoring is required to clarify the association between HF and AF in this subset of patients. Nevertheless, the overall monitoring strategy in our cohort is consistent with current clinical practice and more than 80% of subjects had some form of long term monitoring assuaging concerns of major bias. Finally, our primary outcome of interest was the presence of functional disability at 90 days. Although this approach is consistent with contemporaneous stroke studies, future studies may include assessment of long-term stroke recurrence and survival.
Conclusion
We found that the LVEF and different HF definitions equally relate to the 90-day functional outcome as well as diagnosis of AF in patients with acute ischemic stroke. These results are useful for researchers conducting outcome studies based on HF metrics in stroke populations.
Supplementary Material
Acknowledgments
Sources of Funding
Dr. Henninger is supported by K08NS091499 from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. McManus is supported through R01HL126911-01A1 and NSF Award #1522052.
Footnotes
Competing Interests: Competing Interests: Dr. Henninger serves on the advisory board of Omniox, Inc. Dr. McManus has received consultation or research funding from Samsung Electronics, Philips Healthcare, FlexCon, Biotronik, Bristol Myers Squibb, Pfizer, Care Evolution; Inventor/Equity Stake: MobileSense Technologies, LLC (NIH SBIR R43 HL135961). Dr. Silver has received funding from his service as surveyor for the Joint Commission, adjudicating stroke outcomes for the Women’s Health Initiative, study section reviewer for the NIH, medicolegal malpractice reviews, and he received honoraria for chapters in Ebix, Medlink, and Medscape. All other authors declare no competing interests.
Ethical approval: Our investigation was approved by our Institutional Review Board, and we were granted a Health Insurance Portability and Accountability Act waiver of informed consent.
Data sharing: The investigators will share anonymized data (with associated coding library) used in developing the results presented in this manuscript upon reasonable request to investigators who have received ethical clearance from their host institution.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 2.Kim WJ, Nah HW, Kim DH, et al. Association between Left Ventricular Dysfunction and Functional Outcomes at Three Months in Acute Ischemic Stroke. J Stroke Cerebrovasc Dis 2016;25:2247–2252. [DOI] [PubMed] [Google Scholar]
- 3.Melgaard L, Gorst-Rasmussen A, Lane DA, et al. Assessment of the CHA2DS2-VASc Score in Predicting Ischemic Stroke, Thromboembolism, and Death in Patients With Heart Failure With and Without Atrial Fibrillation. JAMA. 2015;314:1030–1038. [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. [DOI] [PubMed] [Google Scholar]
- 5.McManus DD, Hsu G, Sung SH, et al. Atrial fibrillation and outcomes in heart failure with preserved versus reduced left ventricular ejection fraction. J Am Heart Assoc 2013;2:e005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 7.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 8.Sandhu RK, Hohnloser SH, Pfeffer MA, et al. Relationship between degree of left ventricular dysfunction, symptom status, and risk of embolic events in patients with atrial fibrillation and heart failure. Stroke. 2015;46:667–672. [DOI] [PubMed] [Google Scholar]
- 9.Bennett JA, Riegel B, Bittner V, et al. Validity and reliability of the NYHA classes for measuring research outcomes in patients with cardiac disease. Heart Lung. 2002;31:262–270. [DOI] [PubMed] [Google Scholar]
- 10.Rostagno C, Galanti G, Comeglio M, et al. Comparison of different methods of functional evaluation in patients with chronic heart failure. Eur J Heart Fail. 2000;2:273–280. [DOI] [PubMed] [Google Scholar]
- 11.Raphael C, Briscoe C, Davies J, et al. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart. 2007;93:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. [DOI] [PubMed] [Google Scholar]
- 13.Milionis H, Faouzi M, Cordier M, et al. Characteristics and early and long-term outcome in patients with acute ischemic stroke and low ejection fraction. Int J Cardiol 2013;168:1082–1087. [DOI] [PubMed] [Google Scholar]
- 14.Dries DL, Exner DV, Gersh BJ, et al. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol 1998;32:695–703. [DOI] [PubMed] [Google Scholar]
- 15.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 16.Hong HJ, Kim YD, Cha MJ, et al. Early neurological outcomes according to CHADS2 score in stroke patients with non-valvular atrial fibrillation. Eur J Neurol 2012;19:284–290. [DOI] [PubMed] [Google Scholar]
- 17.Ntaios G, Lip GY, Makaritsis K, et al. CHADS(2), CHA(2)S(2)DS(2)-VASc, and longterm stroke outcome in patients without atrial fibrillation. Neurology. 2013;80:1009–1017. [DOI] [PubMed] [Google Scholar]
- 18.Liu R, Yang X, Li S, et al. Modified CHADS2 and CHA2DS2-VASc scores to predict atrial fibrillation in acute ischemic stroke patients. J Clin Neurosci 2018 [DOI] [PubMed] [Google Scholar]
- 19.Adams HP Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 20.Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014;13:429–438. [DOI] [PubMed] [Google Scholar]
- 21.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. [DOI] [PubMed] [Google Scholar]
- 22.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 23.Hays AG, Sacco RL, Rundek T, et al. Left ventricular systolic dysfunction and the risk of ischemic stroke in a multiethnic population. Stroke. 2006;37:1715–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Echouffo-Tcheugui JB, Erqou S, Butler J, et al. Assessing the Risk of Progression From Asymptomatic Left Ventricular Dysfunction to Overt Heart Failure: A Systematic Overview and Meta-Analysis. JACC Heart Fail. 2016;4:237–248. [DOI] [PubMed] [Google Scholar]
- 25.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–76. [DOI] [PubMed] [Google Scholar]
- 26.De Caterina R, Camm AJ. What is 'valvular' atrial fibrillation? A reappraisal. Eur Heart J 2014;35:3328–3335. [DOI] [PubMed] [Google Scholar]
- 27.Haeusler KG, Laufs U, Endres M. Chronic heart failure and ischemic stroke. Stroke. 2011;42:2977–2982. [DOI] [PubMed] [Google Scholar]
- 28.Appelros P, Nydevik I, Viitanen M. Poor outcome after first-ever stroke: predictors for death, dependency, and recurrent stroke within the first year. Stroke. 2003;34:122–126. [DOI] [PubMed] [Google Scholar]
- 29.Ois A, Gomis M, Cuadrado-Godia E, et al. Heart failure in acute ischemic stroke. J Neurol 2008;255:385–389. [DOI] [PubMed] [Google Scholar]
- 30.Burkot J, Kopec G, Pera J, et al. Decompensated Heart Failure Is a Strong Independent Predictor of Functional Outcome After Ischemic Stroke. J Card Fail. 2015;21:642–646. [DOI] [PubMed] [Google Scholar]
- 31.Byun JI, Jung KH, Kim YD, et al. Cardiac function and outcome in patients with cardioembolic stroke. PLoS One. 2014;9:e95277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdul-Rahim AH, Fulton RL, Frank B, et al. Associations of chronic heart failure with outcome in acute ischaemic stroke patients who received systemic thrombolysis: analysis from VISTA. Eur J Neurol 2015;22:163–169. [DOI] [PubMed] [Google Scholar]
- 33.Scherbakov N, Haeusler KG, Doehner W. Ischemic stroke and heart failure: facts and numbers. ESC Heart Fail. 2015;2:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


