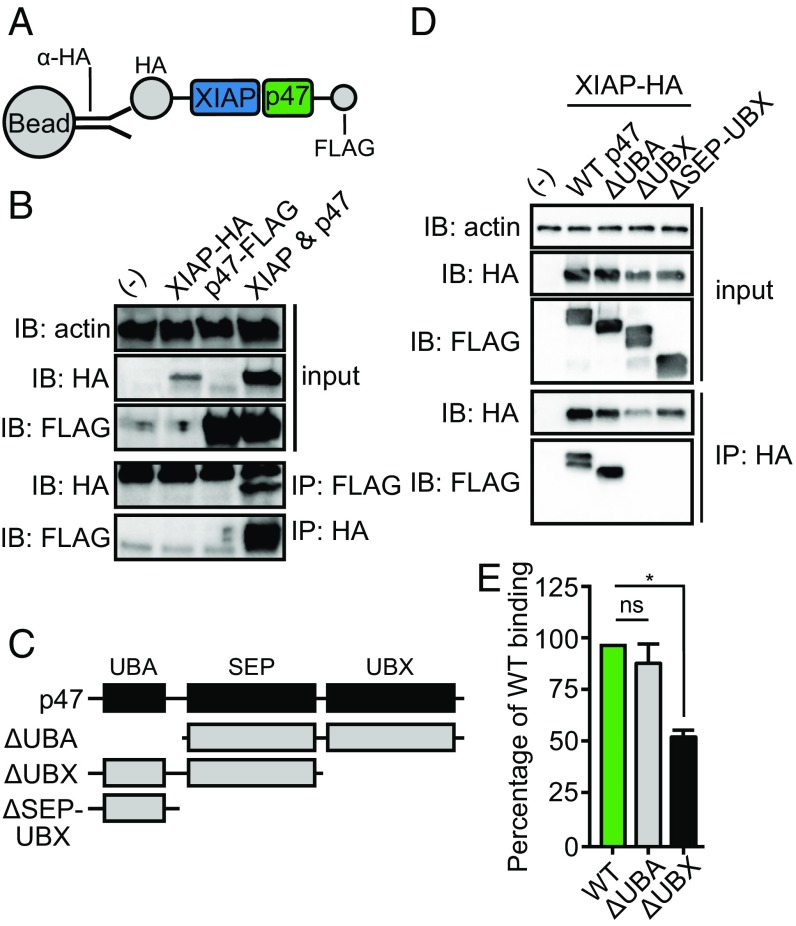
Fig. 2.
The ubiquitin-like (UBX) domain of p47 is required for interaction with XIAP. (A and C) Recombinant tick xiap-hemagglutinin (HA) and p47-FLAG constructs were generated and transfected into human embryonic kidney (HEK) 293T cells to demonstrate that (B) p47-FLAG bound XIAP-HA (D) through the UBX domain. Western blots are representative of at least two independent experiments. (E) 50 ng wild-type (WT) recombinant p47-His, Δ ubiquitin-binding domain (UBA) p47-His, and ΔUBX p47-His were incubated in triplicate with 5 μM XIAP-GST in an ELISA-based binding experiment. The binding signal was normalized to WT p47-His binding (expressed as 100% binding) and is represented as an average of three technical replicates ± SEM. Statistical significance was evaluated by a one-way ANOVA followed by a Tukey post hoc test. Two independent experiments were performed. IB, immunoblot; IP, immunoprecipitation; ns, not significant; *P < 0.05; SEP, shp1, eyes closed, p47 domain; (-) mock transfection.