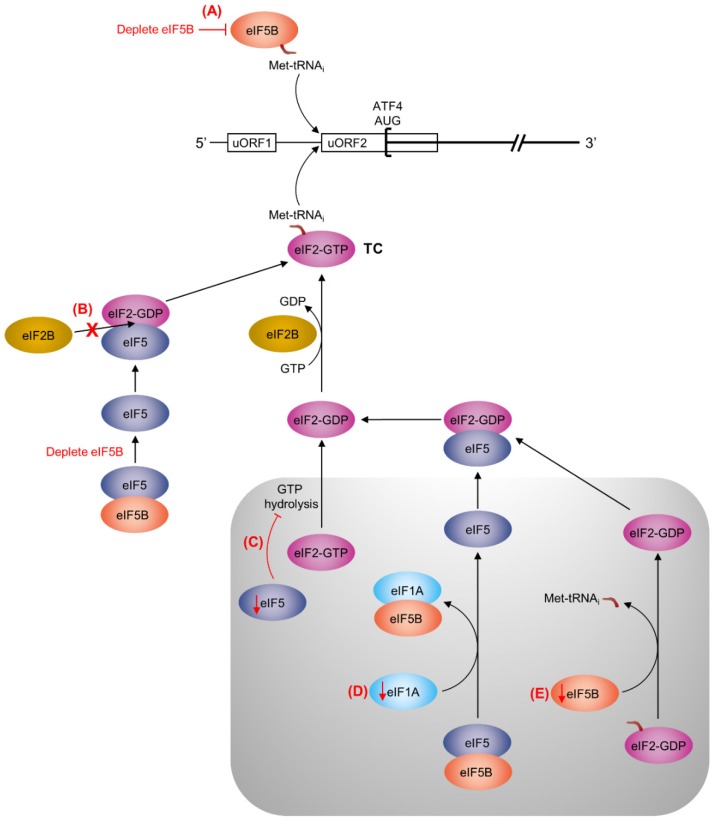
Figure 5.
Possible mechanisms for uORF2-mediated repression of ATF4 translation by eIF5B, eIF1A, and eIF5. The ATF4 mRNA is represented as a horizontal black line, with uORF1 and uORF2 represented as rectangles and the ATF4 start codon represented as a square bracket. In the established mechanism of ATF4 repression, translation re-initiation will tend to occur at the uORF2 start codon when ternary complex (TC) is abundant; however, when TC abundance is decreased (e.g., by phosphorylation of eIF2α), re-initiation will be delayed, allowing the ribosome to bypass uORF2 and initiate translation of ATF4 [24]. Here, we propose several potential mechanisms (A–E, highlighted in red), for uORF2-mediated repression of ATF4 by eIF5B (light orange), eIF5 (dark blue), and eIF1A (light blue). The pre-initiation complex (PIC) is represented by the grey box. Red arrows indicate a decrease of eIF5, eIF1A, or eIF5B. Note that this is not meant to be an exhaustive list and that any one (or any combination) of these mechanisms might be at play. (A) eIF5B might deliver Met-tRNAi to uORF2, providing an alternative to eIF2-GTP (pink). Depletion of eIF5B would consequently decrease translation initiation at the uORF2 start codon, increasing the translation of ATF4. (B) As eIF5B interacts with eIF5 [26], depletion of eIF5B could lead to an increase in free eIF5. Available eIF5 can form a complex with eIF2-GDP that can prevent the interaction of eIF2 with eIF2B (yellow), slowing TC re-formation [33,34]. (C) Depletion of eIF5 would inhibit GTP hydrolysis by eIF2 [33,35,36,37], preventing its release from the PIC and, subsequently, TC re-formation. (D) Depletion of eIF1A would prevent the displacement of eIF5B from eIF5 [26], inhibiting the release of eIF5 and eIF2 from the PIC and, thus, inhibiting TC re-formation. (E) Depletion of eIF5B would inhibit the displacement of eIF2-GDP from Met-tRNAi [33,35,36,37], thus inhibiting TC re-formation.