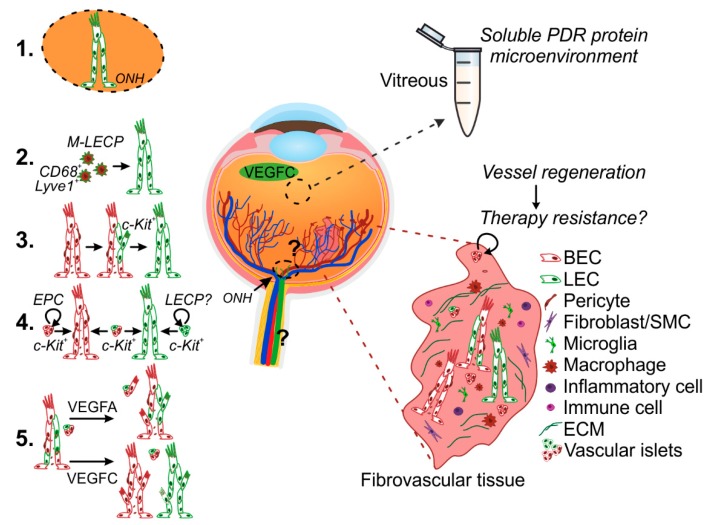
Figure 2.
Potential mechanisms of pathological lymphatic vascular formation in PDR. Lymphatic vessel formation was recently discovered in the ischemia- and inflammation-induced neovascularization occurring in PDR [2,45]. The vitreous fluid collected during vitrectomy reproduces the soluble microenvironment of growth factors, cytokines, and metabolites within the posterior eye chamber and is utilized to indirectly investigate the ongoing pathological processes in the diabetic retina. The patient-derived fibrovascular tissue, excised during vitreoretinal surgery, provides invaluable material for understanding PDR pathophysiology in the complexity of the live human tissue microenvironment. The complex ischemic and inflammatory PDR microenvironment, including VEGFC detected in vitreous, can drive PDR lymphatic vascular formation. Lymphatic vascular formation is essentially achieved through pathological lymphangiogenesis from pre-existing lymphatic vessels and/or de novo, lymph-vasculogenesis. The question on whether the human optic nerve and retinal regions include yet uncovered lymphatic vasculature remains to be investigated (1). Macrophages which have the ability to transform into LECs (M-LECPs) can contribute to de novo lymphatic vascular formation (2). Alternatively, lymphatic neovascularization could occur through trans-differentiation from blood endothelial cells (BECs) (3) or through LEC fate induction in resident or incoming EPCs (4). Sphere-like vascular cell structures interspersed within the PDR fibrovascular tissues could be the vessel primordia for neo (lymph) vascularization. This could also provide a mechanism for vessel regeneration and thus be responsible of re-vascularization after treatment (therapy resistance). The abnormal PDR neovasculature displays heterogeneous population of abnormally differentiated vessels [45]. Ex vivo culture led to heterogeneous vessels and islet sprouting in presence of VEGFA, while VEGFC promoted segregation of the heterogeneous vasculature into separate vessels (5) [45]. More in-depth studies are needed in order to understand the signals and mechanisms involved in the pathological neovascularization observed in PDR. ONH, optic nerve head; BEC, blood endothelial cell; LEC, lymphatic endothelial cell; SMC, smooth muscle cell; LEC-P, LEC-precursor; M-LECP, macrophage-derived LEC-P.