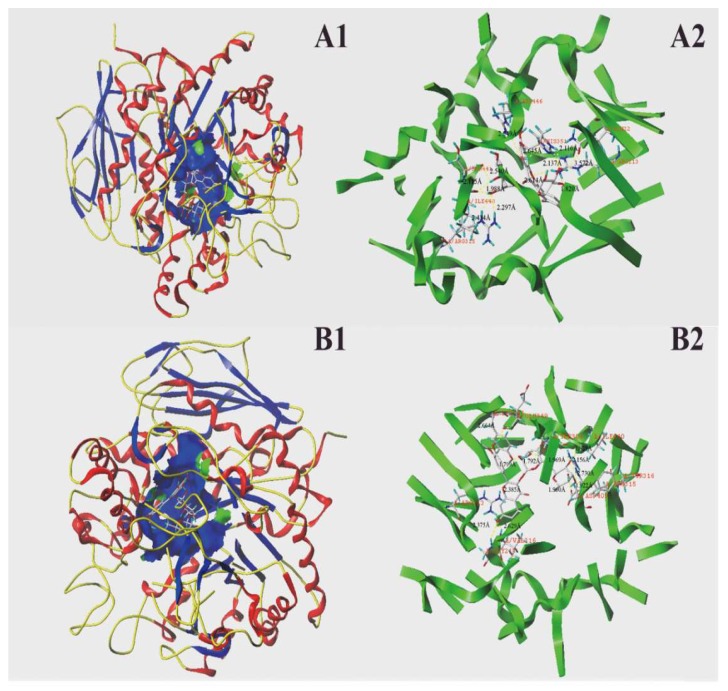
Figure 7.
Molecular docking of two phenolic compounds with α-glucosidase. Two phenolic compounds were inserted into the hydrophobic cavity of α-glucosidase (blue) in the surface structure ((A1), rutin; (B1), isorhamnetin-3-O-rutinoside). Interactions between two phenolic compounds and amino acid residues in the active site of α-glucosidase: rutin conformation (A2), and isorhamnetin 3-O-rutinoside conformation (B2), with residues in the active sites of the α-glucosidase, respectively. The dashed line stands for hydrogen bonds.