Abstract
The importance of zinc for male fertility only emerged recently, being propelled in part by consumer interest in nutritional supplements containing ionic trace minerals. Here, we review the properties, biological roles and cellular mechanisms that are relevant to zinc function in the male reproductive system, survey available peer-reviewed data on nutritional zinc supplementation for fertility improvement in livestock animals and infertility therapy in men, and discuss the recently discovered signaling pathways involving zinc in sperm maturation and fertilization. Emphasis is on the zinc-interacting sperm proteome and its involvement in the regulation of sperm structure and function, from spermatogenesis and epididymal sperm maturation to sperm interactions with the female reproductive tract, capacitation, fertilization, and embryo development. Merits of dietary zinc supplementation and zinc inclusion into semen processing media are considered with livestock artificial insemination (AI) and human assisted reproductive therapy (ART) in mind. Collectively, the currently available data underline the importance of zinc ions for male fertility, which could be harnessed to improve human reproductive health and reproductive efficiency in agriculturally important livestock species. Further research will advance the field of sperm and fertilization biology, provide new research tools, and ultimately optimize semen processing procedures for human infertility therapy and livestock AI.
Keywords: fertilization, sperm, capacitation, zinc, proteasome, fertility
1. Introduction—Encyclopedism of Biological Zinc
Zinc (Zn) is one of the most highly abundant elements on earth, an essential micronutrient to all things living, typically occurring as a divalent cation metal with moderate reactivity and reducing properties. Essential biological roles of zinc include signaling, enzymatic activities, regulation of normal growth and sexual maturation, digestion, homeostasis of central nervous system, and mitochondrial oxidative stress [1,2]. Conversely, zinc imbalance or altered zinc-signaling accompanies pathologies including but not limited to Alzheimer’s disease [3,4,5], blindness, cancer, digestive ailments, growth retardation, and inflammation [6]. While ancient Etruscans and Romans may have already recognized medicinal properties of zinc salts [7], its biological importance was only fully realized in the 19th century, and it entered the mainstream human medicine hundred years later, when the first studied were conducted on dwarfism, human zinc deficiency, and general importance of zinc as a growth factor [8].
Cells of all organisms, ranging from E. coli to mammals, tightly regulate free zinc ion (Zn2+) distribution, even though its toxicity is relatively low [9,10]. In humans, nearly 90% of Zn2+ is found in the muscle and bone [11]. Other organs containing significant concentrations of Zn2+ are the prostate, liver, gastrointestinal tract, kidney, skin, lung, brain, heart, and pancreas [12,13,14]. Homeostasis of Zn2+ is important for survival and fitness; thus, when Zn2+ is consumed in excess, it is important for the body to handle its surplus [15]. Upon ingestion and absorption through the small intestine, the redistribution of Zn2+ occurs via the serum, where Zn2+ is bound predominantly to albumin (major binding protein for up to 60% of Zn2+); the remaining Zn2+ is bound predominantly to 12 other proteins, including α2-macroglobulin, transferrin, ceruloplasmin, IgG, IgA, IgM, complement C4, haptoglobin, and prealbumin [16,17]. Serum Zn2+ accounts for only ~0.1% of bodily Zn2+ [18]. Further, there is no known specialized Zn2+ storage system in the body, and therefore only the daily intake of Zn2+ will ensure steady availability [17].
On the cellular level, 30–40% of Zn2+ localizes in the nucleus, while 50% is stored in the cytoplasm and the rest is associated with membranes [19]. There are two families of proteins that are responsible for the movement of Zn2+ through biological membranes, thus exercising sustained homeostatic control. These include zinc-importer (ZIP; Zrt-, Irt-like) family proteins that transport Zn2+ into the cytosol and the zinc transporter (ZnT) family proteins transporting Zn2+ out of the cytosol [20]. Completion of human genome sequencing identified 14 members of ZIP (designated ZIP1–14) and 10 members ZnT (designated ZnT1–10) [20] families. Few studies have inspected major tissues for the expression patterns of ZnTs in humans [21], and the expression of ZIPs during spermatogenesis is only known in mice [22]. Once Zn2+ enters a cell by ZnTs and ZIPs, it becomes sequestered within the endoplasmic reticulum, mitochondria, and Golgi, or other cell type-specific membrane bound vesicular structures, also called zincomsomes [23,24]. Cytosolic Zn2+ complexing with cytosolic proteins maintains the concentration of free cytosolic Zn2+ within range between picomoles and nanomoles, depending on the cell type [9,25,26,27]. Up to 20% of cytosolic Zn2+ is bound by the apoprotein thionein, to form metallothionein (MT). MTs are small ubiquitous proteins (6–7 kDa) that are rich in cysteine that can complex transition metal ions [28,29]. One molecule of MT can bind up to seven Zn2+, buff excess Zn2+, and supply such cation under Zn2+ deficiency states [30,31].
Limited information exists on the regulation of Zn2+ homeostasis in reproductive system. In female gametes, Zn2+ plays a gatekeeping role in regulating meiotic resumption [32,33,34]. A novel phenomenon of Zn2+ release from the mammalian oocyte at fertilization was recently reported [33,35]; inspiring some of the work on male gametes that will be discussed later. The importance of Zn2+ for male fertility only emerged recently, propelled in part by consumer interest in nutritional supplements containing ionic trace minerals. Here, we review properties, biological roles, and cellular mechanisms that are relevant to zinc function in the male reproductive system, survey available peer-reviewed data on nutritional zinc supplementation for fertility improvement in livestock animals and infertility therapy in men, and discuss recently discovered signaling pathways involving zinc in sperm maturation and fertilization.
2. Zinc-Interacting Sperm Proteins
In eel, zinc is necessary for the sustenance of germ cells and spermatogenesis progression, with N,N,N′,N′-tetrakis(2-pyridinylmethyl)-1,2-ethanediamine (TPEN)-induced Zn deficiency causing germ cell apoptosis [36]. Zinc transporters have been examined in rat, specifically MT I & II in spermatocytes [37] as well as tesmin, a testis-specific metallothionein-like protein [38]. Further, ZnT-7 in mouse testis may supply spermatogenesis-required zinc [39]. Zn2+ transporter ZIP9 serves as a membrane-associated receptor interacting with G-protein Gnα11 to mediate the non-classical testosterone signaling cascade in murine spermatogenic GC-2 cells [40]. Further, the spontaneous Ca2+ oscillations that were observed in spermatogenic cells seem to be modulated by Zn2+ [41]. Zinc ions begin to colonize spermatogenic cells during the final stages of spermatid differentiation when they are incorporated in the nucleus [42] and nascent outer dense fibers (ODF) [43,44]. Additional Zn2+ is incorporated into the nucleus at ejaculation [45]. Nuclear Zn2+ associates with protamines and forms zinc bridges, most likely through imidazole groups of histidine and thiols of cysteine [46], as proposed by Bjorndahl and Kvist, to stabilize the sperm chromatin structure [47,48]. These authors showed that a rapid sperm chromatin decondensation can be induced by Zn2+ chelation with 2,2′,2′′,2′′′-(Ethane-1,2-diyldinitrilo) tetraacetic acid (EDTA), causing the disruption of the protamine zinc bridges [49,50,51]. In the sperm flagellum, Zn2+ is bound to sulfhydryl groups of ODF protein cysteine groups, to protect the nascent flagellum from premature oxidation [52]. During epididymal transit, Zn2+ is selectively removed from the flagellum by a 160 kDa protein, enabling the oxidation of sulfhydryl groups and stiffening the ODF to support progressive motility [52]. High concentrations of Zn2+ have been found in the acrosome [53] and proteolytic conversion of proacrosin to acrosin is inhibited by Zn2+ [54,55], as it probably involves Zn-dependent metalloproteinases. Zinc ions also associate with sperm membranes, where they interact with lipoproteins and membrane-bound metalloproteins in which they react with sulfhydryl groups of cysteine and therefore fulfill a membrane stabilizing function [56,57]. The active removal of Zn2+ is therefore a prerequisite for the completion of sperm capacitation [53], a complex structural and molecular remodeling event that endows spermatozoa within the female reproductive tract with the ability to fertilize. High concentrations of Zn2+ (100 µM) reduce sperm motility in a reversible manner [58]. Initiation of motility following ejaculation [59] and the increased motility of the capacitation-induced sperm hyperactivation are both dependent upon intracellular alkalinization [60].
Additional Zn2+ becomes incorporated into spermatozoa during ejaculation [21,45] where it is believed to have protective function in terms of sperm chromatin decondensation [47,48], sperm motility and metabolic inhibition [58,61], membrane stabilization [56], and antioxidant activity [62,63]. As Zn2+ becomes incorporated into spermatozoa upon mixing with seminal fluid, there are also seminal fluid Zn-interacting proteins competitively binding free Zn2+. In humans, a bulk of seminal fluid Zn2+ is bound to high and low molecular weight ligands derived from prostatic and vesicular secretions [64,65,66,67]. Among them, semenogelins participate in the formation of coagulum, to prevent the retrograde flow of semen deposited in the female tract. Prostasomes, small, exosome-like lipoprotein vesicles are the main zinc-binding partners in human seminal fluid [68,69]. Zinc-binding proteins have also been found in seminal fluid of boar [70] and dog [71,72], and are designated as ZnBP1–6.
3. Zinc-Containing Sperm Proteins
Zinc-containing proteins, commonly known as metalloproteins, are capable of binding one or more Zn2+, usually as a requirement for their biological activity. Human genome sequencing and combined proteomic approaches independently identified 1684 proteins in the human proteome as zinc-containing proteins [73]. Metalloproteins can be further divided into three groups, i.e., (i) metalloenzymes, (ii) metallothioneins, and (iii) gene regulatory proteins [19,74]. Metallothioneins have been discussed in the previous section. Gene regulatory proteins are nucleoproteins that are directly involved with the replication and transcription of DNA. Such DNA binding proteins can be further categorized into three structurally distinct groups, containing: (i) zinc fingers, (ii) zinc clusters, or (iii) zinc twists [75]. Spermatozoa may have limited use for gene regulatory proteins since they are transcriptionally silent; however, these proteins, as, for instance, protamine P2 (discussed earlier) are used heavily for DNA condensation, packaging, and transcriptional suppression [76]. The majority of this section will therefore be dedicated to zinc-containing metalloenzymes, which play a vital role in sperm function.
More than 300 enzymes have been identified that require Zn2+ for their function [19], representing more than 50 different enzyme types. Zn2+ is the only metal that is encountered in all six classes of enzymes, (i.e., oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases). This can be attributed to two properties of Zn2+: (i) relatively low toxicity when compared to other transition metals [77] and (ii) stable association and coordination flexibility with macromolecules [78]. Zn2+ fulfills three functions in the Zn-enzymes: (i) catalytic, (ii) co-active (co-catalytic), and (iii) structural [79]. Catalytic Zn2+ takes part directly in enzyme catalysis. Co-active Zn2+ enhances or diminishes catalytic function in conjunction with catalytic Zn2+, but it is not indispensable for catalytic function [79]. Structural Zn2+ is required for stabilization of the quaternary structure of oligomeric enzymes.
Matrix metalloproteinases (MMPs) belong to a family of zinc-dependent endopeptidases, which are involved in the degradation of extracellular matrix proteins. Since the first discovery of MMPs in the early 1960s, MMPs have grown in number and at least 28 species have been identified to this date; for subtype categorization, distribution, and substrate specificities, see review by Cui et al. [80]. Structurally, a typical MMP contains a propeptide, a catalytic metalloproteinase domain, a linker peptide (hinge region), and a hemopexin domain [80]. The catalytic domain contains two Zn2+ (catalytic and structural) and up to three calcium ions (Ca2+), which stabilize the structure. The cysteine rich region in propeptide chelates the catalytic Zn2+, keeping MMPs in an inactive zymogen form [81]. MMP2 and MMP9, also referred to as Gelatinase-A and Gelatinase-B, were described in human seminal fluid [82,83] and canine epididymal fluid and seminal fluid [84,85]. Furthermore, MMP2 was found to be localized in acrosomal and tail region of normal morphological ejaculated human and canine spermatozoa, while MMP9 was localized in the tail region [85,86]. High levels of MMP2 are associated with high (70%) motility and significantly elevated levels of MMP9 are observed in semen samples with low sperm count [85]. Ferrer at al. [87] demonstrated that MMP2 together with acrosin were confined to the inner acrosomal membrane of epididymal bull sperm and thus introducing the possibility of their cooperation in enzymatic digestion of the oocyte zona pellucida (ZP) during penetration. The regulation of said MMPs by zinc ion fluxes associated with sperm capacitation is currently under investigation. Kratz et al., [88] demonstrated that the levels of seminal MMP2 and MMP9 are correlated with oxidative stress in men, making this a potential diagnostic tool for semen quality/male infertility. Finally, Atabakhsh et al. [89] noticed a positive correlation between seminal fluid MMP2 activity and sperm count, as well as fertilization and embryo quality in couples undergoing assisted reproductive therapy (ART) by intracytoplasmic sperm injection (ICSI), offering a potential predictor of ICSI outcome.
Superoxide dismutases (SOD) are metalloenzymes that are responsible for dismutating the superoxide anion (O2−), to hydrogen peroxide (H2O2), and oxygen (O2) [90]. Three isoforms have been reported in mammals: (i) the cytosolic dimeric Cu/Zn-SOD (SOD1), (ii) the mitochondrial matrix Mn-SOD (SOD2), and (iii) the secretory tetrameric extracellular SOD (EC-SOD/SOD3) [91]. It was shown earlier that both seminal fluid and spermatozoa contain SOD activity [92,93,94,95,96,97,98,99], of which 75% was attributed to SOD1, which is also the main SOD isoform in spermatozoa SOD. The SOD activity in spermatozoa is several-fold higher than SOD activity levels that were previously measured in more than 50 different human somatic cell types [98]. O’Flaherty et al. [94] suggested an important role of superoxide anion in sperm hyperactivation and capacitation; therefore, an adequate balance between superoxide radical generation and dismutation is vital for proper function of spermatozoa, as implicated by Sikka [100].
Another significant group of Zn2+ containing proteins of spermatozoa are sorbitol dehydrogenases that convert sorbitol to fructose, and endow spermatozoa with and have been correlated to motility [101]. Lactate dehydrogenase isoenzyme (LDH-X, LDH-C4) also has been reported to have relationship with sperm motility [102,103,104]. It was shown at least in mice that the inhibition of LDH-C4 blocked sperm capacitation [105]. We previously reported the presence of a ring finger ubiquitin ligase homologous to UBR7 in round spermatids and spermatozoa [106], and implicated this zinc finger containing enzyme in spermiogenesis and possibly in the proteolytic degradation of the ZP at fertilization [107]. Angiotensin converting enzyme (ACE), yet another important Zn2+ containing protein, has been reported in testis, epididymis, and spermatozoa of stallion, boar, and man [108,109,110,111,112,113,114]. Several roles in reproduction have been proposed for ACE, including spermatogenesis [115], sperm capacitation [116,117], and sperm-ZP binding [118]. Alkaline phosphatase (ALP), a homodimeric enzyme containing two Zn2+ and one Mg2+ is present in mammalian seminal fluid [119,120] and spermatozoa [121]. The precise role of ALP in reproduction remains to be discovered, though it may serve as a decapacitating factor [122]. Additional Zn2+ containing proteins that are found in the spermatozoa include fructose-bisphosphate aldolases [123], of which class-II possesses Zn2+ [124], and alcohol dehydrogenase present in human testis and spermatozoa [125,126]. The ADAM (A Disintegrin and Metalloproteinase) protein family plays a role in multiple events leading up to fertilization, including gamete migration and sperm reservoir interactions, sperm-oocyte ZP binding, and sperm-oocyte plasma membrane adhesion and fusion (see review [127]). Noteworthy, proteins in this family possess metalloproteinase domains with Zn2+ [128]. The metalloproteinase domain, however, is cleaved during epididymal transit and only the disintegrin domain remains in mature spermatozoon [127]. Altogether, it is likely that the zinc-interacting proteome plays varied and often essential roles in the regulation of sperm homeostasis and fertilizing ability. Rather than a complete list of zinc-containing proteins, we focused on proteins that are well characterized. We are aware that there are many zinc-containing proteins to be characterized in spermatozoa.
4. Zinc as a Regulator of Sperm Capacitation and Fertilization
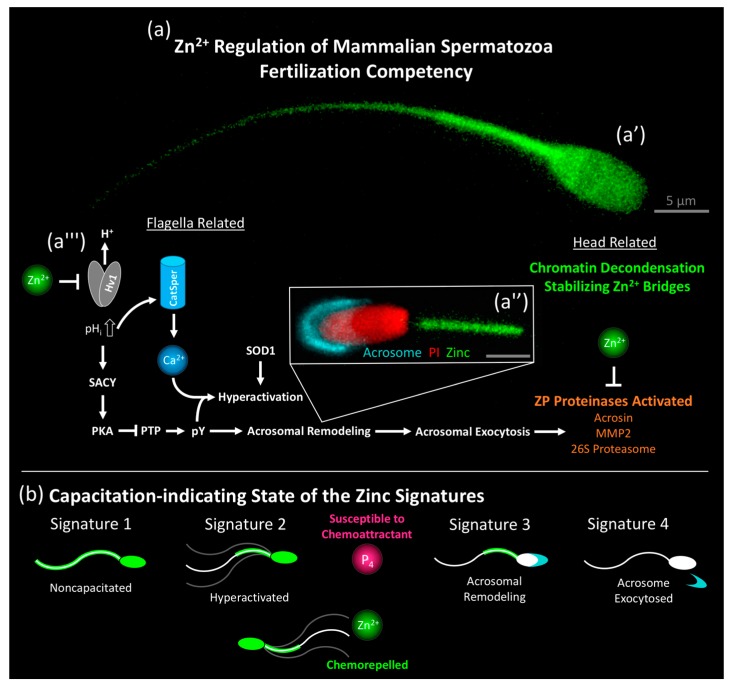
Zinc ions play a vital role in sperm capacitation, regulating key events that are responsible for fertilization competency (summarized in Figure 1a). As much as the Ca2+ influx was understood as key for capacitation, today it is understood that the Zn2+ efflux is the gatekeeper to this important Ca2+ influx [129,130,131,132]. In the following discussion of sperm capacitation, it is important to note the contrasting definitions of sperm capacitation (physiological vs. biochemical) [133] and we will discuss it strictly from the earlier in its original definition (the acquisition of the capacity to fertilize [134]). Prior to the discovery of the sperm capacitation state-reflecting Zn signatures in higher order mammals (boar, bull, and human) [135], there was a noticeable paucity of pivotal discoveries in sperm capacitation translatable from rodent models to humans [136]. Much of this was criticized as a lack of in vivo or minimal inclusion of an in vitro female component in sperm capacitation studies; however, a critical review of literature suggests that this could be due to subtle but vivid differences in the study models and/or experimental design. This includes species differences in attaining intracellular alkalinization [137], thus regulating Ca2+ entry (solely the Na+-dependent Cl−/HCO3− exchanger [138] and possibly the sperm-specific Na+/H+ exchanger sNHE [139] in murine; hydrogen voltage-gated channel, Hv1 expressed by HVCN1 in humans [140]), as well as a result of using epididymal spermatozoa (as opposed to ejaculated). Both of these factors have a notable impact on the Zn signature and result in studies that do not mimic the physiology of ejaculated human semen.
Figure 1.
Summary of Zinc (Zn) signatures and free zinc ion (Zn2+) regulation of the fertilization competency of mammalian spermatozoa. (a) Super-resolution images of the non-capacitated boar sperm Zn signature 1 (a’) and acrosome-remodeled sperm Zn signature 3 (a'') acquired by the Leica TCP SP8 stimulated emission depletion (STED) microscope (free zinc ions in green, outer acrosomal membrane in cyan, remodeled sperm head plasma membrane in red; scale bars in gray: 5 μm). (a''') High Zn2+ concentration (2 mM) negatively regulates proton channel Hv1, responsible for the rise of intracellular pH, facilitating: (1) Ca2+ entry via CatSper and (2) protein tyrosine phosphorylation (pY), triggered by activation of soluble sperm adenylyl cyclase (SACY), increasing intracellular cAMP, activating protein kinase A (PKA) and phosphorylating protein tyrosine phosphatases (PTP) to an inactive state. For general capacitation pathway, review see Kerns et al., [144]). Following acrosome remodeling and exocytosis, zona pellucida (ZP) proteinases (acrosin, MMP2, and the 26S proteasome) implicated in endowing the spermatozoon with the ability to penetrate the ZP are activated. Zn2+, abundantly present in the fertilizing sperm triggered oocyte zinc shield, negatively regulates proteinase activities of spermatozoa bound to the zona or present in the perivitelline space, de-capacitating spermatozoa and serving as a newly proposed anti-polyspermy defense mechanism. (b) Capacitation-indicating state of the zinc signatures. Signature 1 spermatozoa are in a non-capacitated state. Signature 2 spermatozoa display hyperactivated motility. Only capacitating spermatozoa susceptible to progesterone (P4) chemoattraction exhibit chemorepulsion by Zn2+. Signature 3 spermatozoa exhibit acrosome remodeling while acrosomal exocytosis reportedly occurs in signature 4.
There is a moderate negative correlation between flagellar Zn2+ content, and sperm global and progressive motility in humans [141]. Chelation of sperm Zn2+ by (2R,3S)-2,3-Bis(sulfanyl)butanedioic acid (DMSA), 2,3-dimercaptopropane-1-sulfonate (DMPS), or dl-penicillamine leads to increased average straight line velocity and progressive sperm motility while decreasing the percentage of nonlinear motile spermatozoa [142]. Though discovered before the importance of Hv1 in sperm motility activation and capacitation surfaced, previous authors believed this Zn2+ removal to be solely associated with the stiffening of the ODF. Voltage-gated proton channel, Hv1 localizes to the sperm flagellum and it is responsible for sperm cytoplasmic alkalinization through transmembrane proton extrusion [61]. Hv1 is asymmetrically positioned, likely providing differing alkalized microenvironments and gradients in relationship to the symmetrically positioned CatSper channels, thereby being responsible for asymmetrical flagellar bending during hyperactivation [143].
The sperm Zn signature is a collective term for four distinct Zn2+ localization patterns that are indicative of the sperm capacitation state [135]. These zinc ion fluxes are associated with key events in the acquisition of fertilization competency, indicating non-capacitated state, hyperactivation, acrosomal modifications, and acrosomal exocytosis (summarized in Figure 1b). These distinct signatures minimally distinguish the sequential sperm capacitation subpopulations, or to the extent that Zn2+ establishes these sequential subpopulations, and thereby is the previously unknown regulatory time clock of sperm capacitation. The decrease in Zn2+ concentration from the ejaculation/deposition site to the site of fertilization could remove sperm Zn2+ simply via concentration gradient differences and the filtering out of seminal fluid, thereby promoting sperm capacitation. Further, it is well understood that the ubiquitin-dependent protease holoenzyme, the 26S proteasome regulates sperm capacitation (review [144]), as well as the Zn2+ flux in boar spermatozoa [135], and participates in sperm acrosomal exocytosis induced by binding to the egg coat in sea urchin [145], bull [146], and human [147] spermatozoa. Besides acrosomal exocytosis, the sperm-borne 26S proteasome has been implicated in egg coat penetration (ascidian, sea urchin [145], and boar [107]). The relationship between 26S proteasome activity and Zn2+ is unclear, though the proteasomal regulatory subunit PSMD14/Rpn11 contains a metalloprotease-like Zn2+ site [148]. Additionally, Zn2+ has been implicated in regulating proteasome-dependent proteolysis in HeLa cells [149]. Contrarily to the high seminal fluid Zn2+ concentrations (2 mM) inhibiting Hv1, lower concentrations (20 and 50 µM) have been implicated in promoting acrosomal exocytosis in sea urchin [150] and bovine [151] spermatozoa during in vitro capacitation. It is believed that Zn2+ interacts with Zn-sensing receptor (ZnR) GPR39 of the G-protein-coupled receptor (GPCR) family that is found in the sperm acrosome. Such interaction stimulates acrosomal exocytosis through epidermal growth factor receptor (EGFR) transactivation and the phosphorylation of phosphoinositide 3-kinase (PI3K) causing acrosomal Ca2+ mobilization [151]. This implicates a multifaceted role of Zn2+ in sperm capacitation and therefore more research will be needed to fully comprehend these contrasting pathways (inhibiting vs. inducing acrosomal exocytosis).
Successful embryo development in mammals depends upon efficient anti-polyspermy defense, preventing the entry of more than one spermatozoon in the oocyte cytoplasm at fertilization, and thus alleviating an embryo-lethal polyploidy. While membrane depolarization and cortical granule exocytosis are regarded as the main barriers to polyspermy, a sperm-induced Zn2+ release from the oocyte cortex, nicknamed the Zn2+ spark, has recently been discovered in mammals [33,35]. Besides the oocyte Zn2+ spark [33], there is also a physiochemical ZP hardening and a 300% Zn2+ increase in the ZP matrix observed when the fertilized oocyte zona becomes refractory to sperm binding in the mouse [35]. Such a proposed new anti-polyspermy defense mechanism is plausible, although the exact mechanism was not known until recently. We now know that Zn2+ is chemorepulsive, possibly overriding the chemoattraction of oocyte-secreted progesterone in capacitated human, mouse, and rabbit spermatozoa [152]. In light of the sperm Zn signature, a polyspermy defense mechanism of newly fertilized oocytes, termed the zinc shield [135], could in fact de-capacitate spermatozoa already bound to the zona or present in the perivitelline space at the time of fertilization. It is likely such hijacking sperm Zn-signaling and de-capacitation complements the blockage of fertilization through traditional anti-polyspermy mechanisms. Zn2+ has been shown to inhibit fertilization when added to bovine in vitro fertilization (IVF) media [153]. In further support of this mechanism, Zn2+ regulates the activity of the proposed sperm-borne ZP lysins, the proteinases [87] implicated in ZP penetration, including acrosin [54,55], 26S proteasome [135,144], and MMP2 (brain) [154], therefore playing a regulatory role in sperm-ZP penetration. Additionally, inhibitors of zinc-dependent metalloproteases hinder sperm passage through the cumulus oophorus during porcine IVF [155].
5. Effect of Zinc Supplementation on Male Fertility
Reduced seminal fluid Zn2+ has been reported in cases of male infertility that are associated with accidental Chernobyl radiation in Ukraine [156], signifying a possible relationship between Zn2+ and male fertility. Indeed, seminal fluid Zn2+ concentration is positively correlated with sperm count and normal sperm morphology [157]. Consequently, Zn2+ supplementation improves sexual dysfunction in rats [158] and uremic men [159], which is likely due to the ability of Zn2+ to increase serum testosterone levels [160]. The negative effect of fatiguing bicycle exercise on thyroid hormone and testosterone levels in sedentary males is likewise prevented with Zn2+ supplementation [161]. Oral Zn2+ supplementation results in increased sperm counts in ram [162] and humans (combined with inclusion of folate; review) [163]. Zinc supplementation also restores superoxide scavenging antioxidant capacity in asthenospermic men [164]. Dietary Zn2+ intake and action on intraprostatic Zn2+ levels remain unknown; however, if such supplementation increases prostatic levels, it could perceivably increase the percent of non-capacitated signature 1 spermatozoa at the time of ejaculation. Such would be beneficial for inhibiting Hv1, warding off premature sperm capacitation. Goat dietary Zn2+ supplementation increases sperm plasma membrane and acrosome integrity, and percent of viable spermatozoa, also increasing seminal fluid SOD, catalase, and glutathione peroxidase activities [63]. Additionally, Cu2+ and Zn2+ dietary co-supplementation to bucks of the Osmanabadi goat breed allowed for them to reach puberty 28–35 days earlier [165]. While Zn2+ supplementation has a positive influence on multiple male reproductive measures and can serve as male infertility therapy under certain circumstances, one study suggests that supplementation over 100 mg/day is associated with a 2.29 relative risk of advanced prostate cancer [166]. The exact mechanism is unclear, as Zn2+ is not generally considered a carcinogen [167]; yet, increased risk is not surprising as zinc becomes cytotoxic at such concentrations and warrants dosage caution.
Few studies have assessed the addition of Zn2+ in media/semen extenders in agriculturally important livestock species propagated by artificial insemination (AI). A highly desirable property of such media is to reduce reactive oxygen species (ROS) that effect sperm function by the oxidation of lipids, proteins, and DNA (review [168]). Notably, Zn2+ supplementation would serve as an antioxidant by scavenging excessive superoxide anions [169], though Zn2+ homoeostasis is important, and too much Zn2+ can act as a prooxidant and cause mitochondrial oxidative stress (review [2]). Some studies have investigated Zn2+ supplementation, however they did so with the inclusion of d-aspartate and coenzyme Q10, without distinguishing which compound positively reduced lipid peroxidation and DNA fragmentation [170] and improved embryo development [171]. Spermatozoa have the capacity for Zn2+ loading [135], to the extent of restoring their pre-capacitation Zn signature, and it seems reasonable that such would reduce premature, pathological sperm capacitation.
6. Spermatotoxicity and Reprotoxicity of High Zinc Contamination and Zinc Deficiency
At high soil levels, Zn2+ is reprotoxic to the terrestrial worm Enchutraeus crypticus [172]. Few studies exist that observe Zn2+ reprotoxicity [173]. ZnCl2 dietary supplementation to both male and female rats at 30 mg/kg/day but not 15 or 7.5 mg/kg/day showed significant reduction in fertility, offspring viability, and body weight of F1 pups; however, it had no effect on litter size (male reprotoxicity alone was not observed) [174]. Nanosized ZnO toxicity induces sea urchin sperm DNA damage, but it does not reduce fertility [175]. A moderate negative correlation (r = −0.426) has been found between total flagellar Zn2+ content and the percentage of morphologically normal spermatozoa in men [141]. Morphologically abnormal spermatozoa actually contained high amounts of Zn2+ (as reported by fluorescent Zn-probe) [135]. Whether such is caused by Zn2+ toxicity or simply a product of defective spermatozoa failing to regulate their ion fluxes is unknown. Dietary Zn2+ deficiency studies in rat have shown reduced relative weight of the testes, epididymides, and seminal vesicles (organ by percentage of body weight) and reduced prostate weight (by organ weight alone). In the same study, the length of the flagellum was reduced on average by 24% in Zn2+ deficient rats, accompanied by a substantial increase in morphologically abnormal spermatozoa (especially abnormal heads, predominantly headless). Some of these defects could be due to abnormal testicular function from modulated fatty acid composition, interrupting essential fatty acid metabolism, where ω-6 polyunsaturated fatty acids were highly enriched [176]. Zn2+ deficiency is known to trigger autophagy in yeast [177], and elevated autophagy rate during spermatogenesis could decrease sperm count during Zn2+ deficiency. In the absence of fertilization, sperm capacitation is a terminal event leading down a rapid path of apoptosis [178], possibly from the overproduction of ROS [179] in an environment with reduced Zn2+. No studies have been performed to observe whether micromolar levels of Zn2+ under capacitation-inducing conditions can prolong sperm lifespan during a fertilization competent state (as opposed to millimolar levels which inhibit sperm Zn2+ flux [135]). If such could be achieved, fertilization would be possible with fewer spermatozoa and especially useful for artificial insemination in livestock as well as human intrauterine insemination (IUI) in place of costly IVF treatments of couples with an oligospermic male partner.
7. Conclusions and Perspectives
Through a variety of pathways, Zn2+ plays a gatekeeping role in male gametes just as it does in those of the female. Prostatic seminal fluid with the highest concentration of Zn2+ found in any bodily fluids plays a crucial role in fending off premature sperm capacitation and it provides antioxidant activity, while lower concentrations of Zn2+ may be a prerequisite for successful acrosomal exocytosis. A list of reviewed Zn-containing and interacting proteins that are involved from spermatogenesis through the final preparatory steps of fertilization is summarized in Table 1. To fully understand the biological role of Zn2+ in male fertility, further research needs to be pursued, especially to fully disclose the Zn-interacting sperm proteome and its place in various cellular pathways controlling male reproductive function. Additionally, dietary and semen media Zn2+ supplementation has been found to be beneficial for male fertility. Collectively, the currently available data already hint at the importance of zinc ions for male fertility, which could be harnessed to improve the reproductive performance of livestock and increase the success rate of human assisted reproductive therapy. Further research will advance the field of sperm and fertilization biology, provide new research tools, and ultimately optimize the semen processing procedures for human infertility therapy and livestock artificial insemination.
Table 1.
General summary of testicular/sperm Zn-containing and interacting proteins reviewed, in order of discussion.
| Protein | Localization 1 | Function 2 | Reference |
|---|---|---|---|
| Metallothionein I & II | Spermatocytes (rat) | Zinc ion binding | [37] |
| Tesmin | Spermatocytes (rat) | Developmental protein | [37] |
| Zinc transporter ZIP9 | Spermatogenic GC-2 cells (rat) | Zinc transmembrane transporter activity | [38] |
| Protamine-2 | Sperm nucleus (man) | Developmental protein, DNA-binding | [42,47,48] |
| Acrosin | Acrosome (man, bull) | Hydrolase, protease, serine protease | [54,55] |
| Semenoglins | Prostatic and vesicular fluids (man) | - | [64,65,66,67] |
| ZnBP1–6 | Seminal fluid (boar, dog) | - | [70,71,72] |
| Matrix metalloproteinase-2 | Seminal fluid, inner acrosomal membrane, flagellum (man, bull) | Hydrolase, metalloprotease, protease | [87] |
| Matrix metalloproteinase-9 | Seminal fluid and flagellum (man, bull) | Hydrolase, metalloprotease, protease | [87] |
| Superoxide dismutase 1, 2, 3 | Mitochondria and seminal fluids (man) | Antioxidant, oxidoreductase | [91,92,93,94,95,96,97,98,99] |
| l-lactate dehydrogenase | Seminal fluids (man) | Oxidoreductase | [102,103,104] |
| Putative E3 ubiquitin-protein ligase UBR7 | Sperm inner acrosomal membrane (boar) | Transferase | [105] |
| Angiotensin converting enzyme | Testis, epididymis, and spermatozoa (man, boar, stallion) | Carboxypeptidase, hydrolase, metalloprotease, protease | [107] |
| Alkaline phosphatase, germ cell type | Seminal fluid and sperm plasma membrane (man, boar) | Alkaline phosphatase | [119,120,121] |
| Putative class-II fructose-bisphosphate aldolase | Spermatozoa (bull) | Lyase | [123] |
| Alcohol dehydrogenase class-3 | Testis, spermatozoa (man) | Oxidoreductase | [125,126] |
| ADAMs | Spermatozoa | Integrin binding, metalloendopeptidase | Review [127] |
| Voltage-gated hydrogen channel 1 | Flagellum (man) | Ion channel | [140] |
| G-protein coupled receptor | Acrosome (bull) | G-protein coupled receptor activity | [151] |
| 26S proteasome non-ATPase regulatory subunit 14 | Inner acrosomal membrane | Hydrolase, metalloprotease, protease | [180] |
1 Including species reported. 2 Known or predicted function.
Acknowledgments
We thank Alexander Jurkevich and Robert Baker of the University of Missouri Molecular Cytology Core in assistance of Zn signature super-resolution imaging. We also thank Ms. Katherine Craighead for clerical support.
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| AI | Artificial insemination |
| Zn | Zinc |
| Zn2+ | Zinc ion |
| ZIP | Zrt- and Irt-like protein |
| ZnT | Zinc transporter |
| MT | Metallothionein |
| TPEN | N,N,N′,N′-tetrakis(2-pyridinylmethyl)-1,2-ethanediamine |
| ODF | Outer dense fibers |
| EDTA | 2,2′,2″,2′′′-(Ethane-1,2-diyldinitrilo)tetraacetic acid |
| ZnBP | Zinc-binding proteins |
| MMP | Matrix metalloproteinase |
| Ca2+ | Calcium ion |
| ZP | Zona pellucida |
| ICSI | Intracytoplasmic sperm injection |
| SOD | Superoxide dismutase |
| LDH | Lactate dehydrogenase |
| ACE | Angiotensin converting enzyme |
| ALP | Alkaline phosphatase |
| ADAM | A disintegrin and metalloproteinase |
| Hv1 | Hydrogen voltage-gated channel 1 |
| DMSA | (2R,3S)-2,3-Bis(sulfanyl)butanedioic acid |
| DMPS | 2,3-dimercaptopropane-1-sulfonate |
| ZnR | Zn-sensing receptor |
| GPCR | G-protein-coupled receptor |
| EGFR | Epidermal growth factor receptor |
| PI3K | Phosphoinositide 3-kinase |
| IVF | In vitro fertilization |
| pY | Protein tyrosine phosphorylation |
| SACY | Soluble sperm adenylyl cyclase |
| PKA | Protein kinase A |
| PTP | Protein tyrosine phosphatase |
| ROS | Reactive oxygen species |
| IUI | Intrauterine insemination |
Author Contributions
All authors contributed to investigation, original draft preparation, review and editing; visualization, K.K.; supervision, P.S.; project administration, P.S.; funding acquisition, P.S. and K.K.
Funding
This review was funded by National Institute of Food and Agriculture (NIFA), U.S. Department of Agriculture (USDA) grant number 2015-67015-23231 (P.S.), USDA NIFA Graduate Fellowship award number 2017-67011-26023 (K.K.), grant number 5 R01 HD084353-02 from National Institutes of Health (NIH) National Institute of Child and Human Development (P.S.), and seed funding from the Food for the 21st Century Program of the University of Missouri (P.S.).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in this review; in the collection, analyses, or interpretation; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Levaot N., Hershfinkel M. How cellular Zn2+ signaling drives physiological functions. Cell Calcium. 2018;75:53–63. doi: 10.1016/j.ceca.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Lee S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxid. Med. Cell. Longev. 2018;2018:9156285. doi: 10.1155/2018/9156285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lovell M.A. A potential role for alterations of zinc and zinc transport proteins in the progression of Alzheimer’s disease. J. Alzheimer’s Dis. 2009;16:471–483. doi: 10.3233/JAD-2009-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szewczyk B. Zinc homeostasis and neurodegenerative disorders. Front. Aging Neurosc. 2013;5:33. doi: 10.3389/fnagi.2013.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prakash A., Bharti K., Majeed A.B. Zinc: Indications in brain disorders. Fundament. Clin. Pharmacol. 2015;29:131–149. doi: 10.1111/fcp.12110. [DOI] [PubMed] [Google Scholar]
- 6.Prasad A.S. Zinc: Role in immunity, oxidative stress and chronic inflammation. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:646–652. doi: 10.1097/MCO.0b013e3283312956. [DOI] [PubMed] [Google Scholar]
- 7.Giachi G., Pallecchi P., Romualdi A., Ribechini E., Lucejko J.J., Colombini M.P., Mariotti Lippi M. Ingredients of a 2,000-y-old medicine revealed by chemical, mineralogical, and botanical investigations. Proc. Natl. Acad. Sci. USA. 2013;110:1193–1196. doi: 10.1073/pnas.1216776110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasad A.S. Discovery of human zinc deficiency: Its impact on human health and disease. Adv. Nutr. 2013;4:176–190. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Outten C.E., O’Halloran T.V. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 10.Beyersmann D., Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals. 2001;14:331–341. doi: 10.1023/A:1012905406548. [DOI] [PubMed] [Google Scholar]
- 11.Wastney M.E., Aamodt R.L., Rumble W.F., Henkin R.I. Kinetic analysis of zinc metabolism and its regulation in normal humans. Am. J. Physiol. 1986;251 Pt 2:R398–R408. doi: 10.1152/ajpregu.1986.251.2.R398. [DOI] [PubMed] [Google Scholar]
- 12.Bentley P.J., Grubb B.R. Effects of a zinc-deficient diet on tissue zinc concentrations in rabbits. J. Anim. Sci. 1991;69:4876–4882. doi: 10.2527/1991.69124876x. [DOI] [PubMed] [Google Scholar]
- 13.He L.S., Yan X.S., Wu D.C. Age-dependent variation of zinc-65 metabolism in LACA mice. Int. J. Radiat. Biol. 1991;60:907–916. doi: 10.1080/09553009114552711. [DOI] [PubMed] [Google Scholar]
- 14.Llobet J.M., Domingo J.L., Colomina M.T., Mayayo E., Corbella J. Subchronic oral toxicity of zinc in rats. Bull. Environ. Contam. Toxicol. 1988;41:36–43. doi: 10.1007/BF01689056. [DOI] [PubMed] [Google Scholar]
- 15.Jones M.M., Schoenheit J.E., Weaver A.D. Pretreatment and heavy metal LD50 values. Toxicol. Appl. Pharmacol. 1979;49:41–44. doi: 10.1016/0041-008X(79)90274-6. [DOI] [PubMed] [Google Scholar]
- 16.Prasad A.S., Oberleas D. Binding of zinc to amino acids and serum proteins in vitro. J. Lab. Clin. Med. 1970;76:416–425. [PubMed] [Google Scholar]
- 17.Scott B.J., Bradwell A.R. Identification of the serum binding proteins for iron, zinc, cadmium, nickel, and calcium. Clin. Chem. 1983;29:629–633. [PubMed] [Google Scholar]
- 18.Rukgauer M., Klein J., Kruse-Jarres J.D. Reference values for the trace elements copper, manganese, selenium, and zinc in the serum/plasma of children, adolescents, and adults. J. Trace Elem. Med. Biol. 1997;11:92–98. doi: 10.1016/S0946-672X(97)80032-6. [DOI] [PubMed] [Google Scholar]
- 19.Vallee B.L., Falchuk K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 20.Lichten L.A., Cousins R.J. Mammalian zinc transporters: Nutritional and physiologic regulation. Ann. Rev. Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 21.Foresta C., Garolla A., Cosci I., Menegazzo M., Ferigo M., Gandin V., De Toni L. Role of zinc trafficking in male fertility: From germ to sperm. Hum. Reprod. 2014;29:1134–1145. doi: 10.1093/humrep/deu075. [DOI] [PubMed] [Google Scholar]
- 22.Croxford T.P., McCormick N.H., Kelleher S.L. Moderate zinc deficiency reduces testicular Zip6 and Zip10 abundance and impairs spermatogenesis in mice. J. Nutr. 2011;141:359–365. doi: 10.3945/jn.110.131318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zalewski P.D., Forbes I.J., Betts W.H. Correlation of apoptosis with change in intracellular labile Zn(II) using zinquin [(2-methyl-8-p-toluenesulphonamido-6-quinolyloxy)acetic acid], a new specific fluorescent probe for Zn(II) Biochem. J. 1993;296 Pt 2:403–408. doi: 10.1042/bj2960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wellenreuther G., Cianci M., Tucoulou R., Meyer-Klaucke W., Haase H. The ligand environment of zinc stored in vesicles. Biochem. Biophys. Res. Commun. 2009;380:198–203. doi: 10.1016/j.bbrc.2009.01.074. [DOI] [PubMed] [Google Scholar]
- 25.Qin Y., Dittmer P.J., Park J.G., Jansen K.B., Palmer A.E. Measuring steady-state and dynamic endoplasmic reticulum and Golgi Zn2+ with genetically encoded sensors. Proc. Natl. Acad. Sci. USA. 2011;108:7351–7356. doi: 10.1073/pnas.1015686108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sensi S.L., Canzoniero L.M., Yu S.P., Ying H.S., Koh J.Y., Kerchner G.A., Choi D.W. Measurement of intracellular free zinc in living cortical neurons: Routes of entry. J. Neurosci. 1997;17:9554–9564. doi: 10.1523/JNEUROSCI.17-24-09554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vinkenborg J.L., Nicolson T.J., Bellomo E.A., Koay M.S., Rutter G.A., Merkx M. Genetically encoded FRET sensors to monitor intracellular Zn2+ homeostasis. Nat. Methods. 2009;6:737–740. doi: 10.1038/nmeth.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tapiero H., Tew K.D. Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed. Pharmacother. 2003;57:399–411. doi: 10.1016/S0753-3322(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 29.Coyle P., Philcox J.C., Carey L.C., Rofe A.M. Metallothionein: The multipurpose protein. Cell. Mol. Life Sci. 2002;59:627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blindauer C.A., Leszczyszyn O.I. Metallothioneins: Unparalleled diversity in structures and functions for metal ion homeostasis and more. Nat. Prod. Rep. 2010;27:720–741. doi: 10.1039/b906685n. [DOI] [PubMed] [Google Scholar]
- 31.Maret W., Krezel A. Cellular zinc and redox buffering capacity of metallothionein/thionein in health and disease. Mol. Med. 2007;13:371–375. doi: 10.2119/2007-00036.Maret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao M.H., Kwon J.W., Liang S., Kim S.H., Li Y.H., Oh J.S., Kim N.H., Cui X.S. Zinc regulates meiotic resumption in porcine oocytes via a protein kinase C-related pathway. PLoS ONE. 2014;9:e102097. doi: 10.1371/journal.pone.0102097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim A.M., Bernhardt M.L., Kong B.Y., Ahn R.W., Vogt S., Woodruff T.K., O’Halloran T.V. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem. Biol. 2011;6:716–723. doi: 10.1021/cb200084y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim A.M., Vogt S., O’Halloran T.V., Woodruff T.K. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat. Chem. Biol. 2010;6:674–681. doi: 10.1038/nchembio.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Que E.L., Duncan F.E., Bayer A.R., Philips S.J., Roth E.W., Bleher R., Gleber S.C., Vogt S., Woodruff T.K., O’Halloran T.V. Zinc sparks induce physiochemical changes in the egg zona pellucida that prevent polyspermy. Integr. Biol. 2017;9:135–144. doi: 10.1039/C6IB00212A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaguchi S., Miura C., Kikuchi K., Celino F.T., Agusa T., Tanabe S., Miura T. Zinc is an essential trace element for spermatogenesis. Proc. Natl. Acad. Sci. USA. 2009;106:10859–10864. doi: 10.1073/pnas.0900602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elgazar V., Razanov V., Stoltenberg M., Hershfinkel M., Huleihel M., Nitzan Y.B., Lunenfeld E., Sekler I., Silverman W.F. Zinc-regulating proteins, ZnT-1, and metallothionein I/II are present in different cell populations in the mouse testis. J. Histochem. Cytochem. 2005;53:905–912. doi: 10.1369/jhc.4A6482.2005. [DOI] [PubMed] [Google Scholar]
- 38.Sugihara T., Wadhwa R., Kaul S.C., Mitsui Y. A novel testis-specific metallothionein-like protein, tesmin, is an early marker of male germ cell differentiation. Genomics. 1999;57:130–136. doi: 10.1006/geno.1999.5756. [DOI] [PubMed] [Google Scholar]
- 39.Chi Z.H., Feng W.Y., Gao H.L., Zheng W., Huang L., Wang Z.Y. ZNT7 and Zn2+ are present in different cell populations in the mouse testis. Histol. Histopathol. 2009;24:25–30. doi: 10.14670/HH-24.25. [DOI] [PubMed] [Google Scholar]
- 40.Shihan M., Chan K.H., Konrad L., Scheiner-Bobis G. Non-classical testosterone signaling in spermatogenic GC-2 cells is mediated through ZIP9 interacting with Gnalpha11. Cell. Signal. 2015;27:2077–2086. doi: 10.1016/j.cellsig.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Gonzalez I., Trevino C.L., Darszon A. Regulation of Spermatogenic Cell T-Type Ca2+ Currents by Zn2+: Implications in Male Reproductive Physiology. J. Cell. Physiol. 2016;231:659–667. doi: 10.1002/jcp.25112. [DOI] [PubMed] [Google Scholar]
- 42.Barney G.H., Orgebin-Crist M.C., Macapinalac M.P. Genesis of esophageal parakeratosis and histologic changes in the testes of the zinc-deficient rat and their reversal by zinc repletion. J. Nutr. 1968;95:526–534. doi: 10.1093/jn/95.4.526. [DOI] [PubMed] [Google Scholar]
- 43.Baccetti B., Pallini V., Burrini A.G. The accessory fibers of the sperm tail. II. Their role in binding zinc in mammals and cephalopods. J. Ultrastruct. Res. 1976;54:261–275. doi: 10.1016/S0022-5320(76)80155-4. [DOI] [PubMed] [Google Scholar]
- 44.Bedford J.M., Calvin H.I. Changes in -S-S- linked structures of the sperm tail during epididymal maturation, with comparative observations in sub-mammalian species. J. Exp. Zool. 1974;187:181–204. doi: 10.1002/jez.1401870202. [DOI] [PubMed] [Google Scholar]
- 45.Bjorndahl L., Kjellberg S., Roomans G.M., Kvist U. The human sperm nucleus takes up zinc at ejaculation. Int. J. Androl. 1986;9:77–80. doi: 10.1111/j.1365-2605.1986.tb00869.x. [DOI] [PubMed] [Google Scholar]
- 46.Porath J., Carlsson J., Olsson I., Belfrage G. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature. 1975;258:598–599. doi: 10.1038/258598a0. [DOI] [PubMed] [Google Scholar]
- 47.Bjorndahl L., Kvist U. Human sperm chromatin stabilization: A proposed model including zinc bridges. Mol. Hum. Reprod. 2010;16:23–29. doi: 10.1093/molehr/gap099. [DOI] [PubMed] [Google Scholar]
- 48.Bjorndahl L., Kvist U. A model for the importance of zinc in the dynamics of human sperm chromatin stabilization after ejaculation in relation to sperm DNA vulnerability. Syst. Biol. Reprod. Med. 2011;57:86–92. doi: 10.3109/19396368.2010.516306. [DOI] [PubMed] [Google Scholar]
- 49.Kvist U. Importance of spermatozoal zinc as temporary inhibitor of sperm nuclear chromatin decondensation ability in man. Acta Physiol. Scand. 1980;109:79–84. doi: 10.1111/j.1748-1716.1980.tb06567.x. [DOI] [PubMed] [Google Scholar]
- 50.Kvist U. Sperm nuclear chromatin decondensation ability. An in vitro study on ejaculated human spermatozoa. Acta physiol. Scand. Suppl. 1980;486:1–24. [PubMed] [Google Scholar]
- 51.Roomans G.M., Lundevall E., Bjorndahl L., Kvist U. Removal of zinc from subcellular regions of human spermatozoa by EDTA treatment studied by X-ray microanalysis. Int. J. Androl. 1982;5:478–486. doi: 10.1111/j.1365-2605.1982.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 52.Henkel R., Baldauf C., Bittner J., Weidner W., Miska W. Elimination of zinc from the flagella of spermatozoa during epididymal transit is important for motility. Reprod. Technol. 2001;10:6. [Google Scholar]
- 53.Andrews J.C., Nolan J.P., Hammerstedt R.H., Bavister B.D. Role of zinc during hamster sperm capacitation. Biol. Reprod. 1994;51:1238–1247. doi: 10.1095/biolreprod51.6.1238. [DOI] [PubMed] [Google Scholar]
- 54.Steven F.S., Griffin M.M., Chantler E.N. Inhibition of human and bovine sperm acrosin by divalent metal ions. Possible role of zinc as a regulator of acrosin activity. Int. J. Androl. 1982;5:401–412. doi: 10.1111/j.1365-2605.1982.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 55.Johnsen O., Eliasson R., Lofman C.O. Inhibition of the gelatinolytic and esterolytic activity of human sperm acrosin by zinc. Acta Physiol. Scand. 1982;114:475–476. doi: 10.1111/j.1748-1716.1982.tb07013.x. [DOI] [PubMed] [Google Scholar]
- 56.Bettger W.J., O’Dell B.L. A critical physiological role of zinc in the structure and function of biomembranes. Life Sci. 1981;28:1425–1438. doi: 10.1016/0024-3205(81)90374-X. [DOI] [PubMed] [Google Scholar]
- 57.Mankad M., Sathawara N.G., Doshi H., Saiyed H.N., Kumar S. Seminal plasma zinc concentration and alpha-glucosidase activity with respect to semen quality. Biol. Trace Elem. Res. 2006;110:97–106. doi: 10.1385/BTER:110:2:97. [DOI] [PubMed] [Google Scholar]
- 58.Riffo M., Leiva S., Astudillo J. Effect of zinc on human sperm motility and the acrosome reaction. Int. J. Androl. 1992;15:229–237. doi: 10.1111/j.1365-2605.1992.tb01343.x. [DOI] [PubMed] [Google Scholar]
- 59.Acott T.S., Carr D.W. Inhibition of bovine spermatozoa by caudal epididymal fluid: II. Interaction of pH and a quiescence factor. Biol. Reprod. 1984;30:926–935. doi: 10.1095/biolreprod30.4.926. [DOI] [PubMed] [Google Scholar]
- 60.Ho H.C., Granish K.A., Suarez S.S. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev. Biol. 2002;250:208–217. doi: 10.1006/dbio.2002.0797. [DOI] [PubMed] [Google Scholar]
- 61.Babcock D.F., Rufo G.A., Jr., Lardy H.A. Potassium-dependent increases in cytosolic pH stimulate metabolism and motility of mammalian sperm. Proc. Natl. Acad. Sci. USA. 1983;80:1327–1331. doi: 10.1073/pnas.80.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bray T.M., Bettger W.J. The physiological role of zinc as an antioxidant. Free Radic. Biol. Med. 1990;8:281–291. doi: 10.1016/0891-5849(90)90076-U. [DOI] [PubMed] [Google Scholar]
- 63.Narasimhaiah M., Arunachalam A., Sellappan S., Mayasula V.K., Guvvala P.R., Ghosh S.K., Chandra V., Ghosh J., Kumar H. Organic zinc and copper supplementation on antioxidant protective mechanism and their correlation with sperm functional characteristics in goats. Reprod. Domest. Anim. 2018;53:644–654. doi: 10.1111/rda.13154. [DOI] [PubMed] [Google Scholar]
- 64.Arver S. Zinc and zinc ligands in human seminal plasma. I. Methodological aspects and normal findings. Int. J. Androl. 1980;3:629–642. doi: 10.1111/j.1365-2605.1980.tb00151.x. [DOI] [PubMed] [Google Scholar]
- 65.Arver S., Eliasson R. Zinc and zinc ligands in human seminal plasma. II. Contribution by ligands of different origin to the zinc binding properties of human seminal plasma. Acta Physiol. Scand. 1982;115:217–224. doi: 10.1111/j.1748-1716.1982.tb07068.x. [DOI] [PubMed] [Google Scholar]
- 66.Arver S. Zinc and zinc ligands in human seminal plasma. III. The principal low molecular weight zinc ligand in prostatic secretion and seminal plasma. Acta Physiol. Scand. 1982;116:67–73. doi: 10.1111/j.1748-1716.1982.tb10600.x. [DOI] [PubMed] [Google Scholar]
- 67.Siciliano L., De Stefano C., Petroni M.F., Vivacqua A., Rago V., Carpino A. Prostatic origin of a zinc binding high molecular weight protein complex in human seminal plasma. Mol. Hum. Reprod. 2000;6:215–218. doi: 10.1093/molehr/6.3.215. [DOI] [PubMed] [Google Scholar]
- 68.Robert M., Gagnon C. Semenogelin I: A coagulum forming, multifunctional seminal vesicle protein. Cell. Mol. Life Sci. 1999;55:944–960. doi: 10.1007/s000180050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vivacqua A., Siciliano L., Sabato M., Palma A., Carpino A. Prostasomes as zinc ligands in human seminal plasma. Int. J. Androl. 2004;27:27–31. doi: 10.1111/j.1365-2605.2004.00441.x. [DOI] [PubMed] [Google Scholar]
- 70.Mogielnicka-Brzozowska M., Wysocki P., Strzezek J., Kordan W. Zinc-binding proteins from boar seminal plasma—Isolation, biochemical characteristics and influence on spermatozoa stored at 4 degrees C. Acta Biochim. Pol. 2011;58:171–177. [PubMed] [Google Scholar]
- 71.Johnson L., Wikström S., Nylander G. The Vehicle for Zinc in the Prostatic Secretion of Dogs. Scand. J. Urol. Nephrol. 1969;3:9–11. doi: 10.3109/00365596909135373. [DOI] [PubMed] [Google Scholar]
- 72.Mogielnicka-Brzozowska M., Strzezek R., Wasilewska K., Kordan W. Prostasomes of canine seminal plasma—Zinc-binding ability and effects on motility characteristics and plasma membrane integrity of spermatozoa. Reprod. Domest. Anim. 2015;50:484–491. doi: 10.1111/rda.12516. [DOI] [PubMed] [Google Scholar]
- 73.Andreini C., Banci L., Bertini I., Rosato A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006;5:196–201. doi: 10.1021/pr050361j. [DOI] [PubMed] [Google Scholar]
- 74.Coleman J.E. Zinc proteins: Enzymes, storage proteins, transcription factors, and replication proteins. Ann. Rev. Biochem. 1992;61:897–946. doi: 10.1146/annurev.bi.61.070192.004341. [DOI] [PubMed] [Google Scholar]
- 75.Vallee B.L., Coleman J.E., Auld D.S. Zinc fingers, zinc clusters, and zinc twists in DNA-binding protein domains. Proc. Natl. Acad. Sci. USA. 1991;88:999–1003. doi: 10.1073/pnas.88.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bianchi F., Rousseaux-Prevost R., Sautiere P., Rousseaux J. P2 protamines from human sperm are zinc -finger proteins with one CYS2/HIS2 motif. Biochem. Biophys. Res. Commun. 1992;182:540–547. doi: 10.1016/0006-291X(92)91766-J. [DOI] [PubMed] [Google Scholar]
- 77.Seiler H.G., Sigel H. Handbook on Toxicity of Inorganic Compounds. Marcel Dekker; New York, NY, USA: 1988. [Google Scholar]
- 78.Vallee B.L., Auld D.S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990;29:5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- 79.Vallee B.L., Auld D.S. Active zinc binding sites of zinc metalloenzymes. In: Birkedal-Hansen H., Werb Z., Welgus H., Van Wart H., editors. Matrix Metalloproteinases and Inhibitors. Fischer; Stuttgart, Germany: 1992. p. 5. [PubMed] [Google Scholar]
- 80.Cui N., Hu M., Khalil R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017;147:1–73. doi: 10.1016/bs.pmbts.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nagase H., Visse R., Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 82.Shimokawa Ki K., Katayama M., Matsuda Y., Takahashi H., Hara I., Sato H., Kaneko S. Matrix metalloproteinase (MMP)-2 and MMP-9 activities in human seminal plasma. Mol. Hum. Reprod. 2002;8:32–36. doi: 10.1093/molehr/8.1.32. [DOI] [PubMed] [Google Scholar]
- 83.Tentes I., Asimakopoulos B., Mourvati E., Diedrich K., Al-Hasani S., Nikolettos N. Matrix metalloproteinase (MMP)-2 and MMP-9 in seminal plasma. J. Assist. Reprod. Genet. 2007;24:278–281. doi: 10.1007/s10815-007-9129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saengsoi W., Shia W.Y., Shyu C.L., Wu J.T., Warinrak C., Lee W.M., Cheng F.P. Detection of matrix metalloproteinase (MMP)-2 and MMP-9 in canine seminal plasma. Anim. Reprod. Sci. 2011;127:114–119. doi: 10.1016/j.anireprosci.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 85.Warinrak C., Wu J.T., Hsu W.L., Liao J.W., Chang S.C., Cheng F.P. Expression of matrix metalloproteinases (MMP-2, MMP-9) and their inhibitors (TIMP-1, TIMP-2) in canine testis, epididymis and semen. Reprod. Domest. Anim. 2015;50:48–57. doi: 10.1111/rda.12448. [DOI] [PubMed] [Google Scholar]
- 86.Buchman-Shaked O., Kraiem Z., Gonen Y., Goldman S. Presence of matrix metalloproteinases and tissue inhibitor of matrix metalloproteinase in human sperm. J. Androl. 2002;23:702–708. [PubMed] [Google Scholar]
- 87.Ferrer M., Rodriguez H., Zara L., Yu Y., Xu W., Oko R. MMP2 and acrosin are major proteinases associated with the inner acrosomal membrane and may cooperate in sperm penetration of the zona pellucida during fertilization. Cell. Tissue Res. 2012;349:881–895. doi: 10.1007/s00441-012-1429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kratz E.M., Kaluza A., Ferens-Sieczkowska M., Olejnik B., Fiutek R., Zimmer M., Piwowar A. Gelatinases and their tissue inhibitors are associated with oxidative stress: A potential set of markers connected with male infertility. Reprod. Fertil. Dev. 2016;28:1029–1037. doi: 10.1071/RD14268. [DOI] [PubMed] [Google Scholar]
- 89.Atabakhsh M., Khodadadi I., Amiri I., Mahjub H., Tavilani H. Activity of Matrix Metalloproteinase 2 and 9 in Follicular Fluid and Seminal Plasma and Its Relation to Embryo Quality and Fertilization Rate. J. Reprod. Infertil. 2018;19:140–145. [PMC free article] [PubMed] [Google Scholar]
- 90.McCord J.M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 91.Marklund S.L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem. J. 1984;222:649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alvarez J.G., Touchstone J.C., Blasco L., Storey B.T. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J. Androl. 1987;8:338–348. doi: 10.1002/j.1939-4640.1987.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 93.Beconi M.T., Francia C.R., Mora N.G., Affranchino M.A. Effect of natural antioxidants on frozen bovine semen preservation. Theriogenology. 1993;40:841–851. doi: 10.1016/0093-691X(93)90219-U. [DOI] [PubMed] [Google Scholar]
- 94.O’Flaherty C., Beconi M., Beorlegui N. Effect of natural antioxidants, superoxide dismutase and hydrogen peroxide on capacitation of frozen-thawed bull spermatozoa. Andrologia. 1997;29:269–275. doi: 10.1111/j.1439-0272.1997.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 95.Kowalowka M., Wysocki P., Fraser L., Strzezek J. Extracellular superoxide dismutase of boar seminal plasma. Reprod. Domest. Anim. 2008;43:490–496. doi: 10.1111/j.1439-0531.2007.00943.x. [DOI] [PubMed] [Google Scholar]
- 96.Strzezek R., Fraser L. Characteristics of spermatozoa of whole ejaculate and sperm-rich fraction of dog semen following exposure to media varying in osmolality. Reprod. Biol. 2009;9:113–126. doi: 10.1016/S1642-431X(12)60021-7. [DOI] [PubMed] [Google Scholar]
- 97.Marti E., Mara L., Marti J.I., Muino-Blanco T., Cebrian-Perez J.A. Seasonal variations in antioxidant enzyme activity in ram seminal plasma. Theriogenology. 2007;67:1446–1454. doi: 10.1016/j.theriogenology.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 98.Peeker R., Abramsson L., Marklund S.L. Superoxide dismutase isoenzymes in human seminal plasma and spermatozoa. Mol. Hum. Reprod. 1997;3:1061–1066. doi: 10.1093/molehr/3.12.1061. [DOI] [PubMed] [Google Scholar]
- 99.Park K., Jeon S., Song Y.-J., Yi L.S.H. Proteomic analysis of boar spermatozoa and quantity changes of superoxide dismutase 1, glutathione peroxidase, and peroxiredoxin 5 during epididymal maturation. Anim. Reprod. Sci. 2012;135:53–61. doi: 10.1016/j.anireprosci.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 100.Sikka S.C. Relative impact of oxidative stress on male reproductive function. Curr. Med. Chem. 2001;8:851–862. doi: 10.2174/0929867013373039. [DOI] [PubMed] [Google Scholar]
- 101.Dhanda O.P., Rao B.R., Razdan M.N. Sorbitol dehydrogenase & hyaluronidase activity in buffalo semen. Indian J. Exp. Biol. 1981;19:286. [PubMed] [Google Scholar]
- 102.Gavella M., Cvitkovic P. Semen LDH-X deficiency and male infertility. Arch. Androl. 1985;15:173–176. doi: 10.3109/01485018508986907. [DOI] [PubMed] [Google Scholar]
- 103.Virji N. LDH-C4 in human seminal plasma and its relationship to testicular function. I. Methodological aspects. Int. J. Androl. 1985;8:193–200. doi: 10.1111/j.1365-2605.1985.tb00834.x. [DOI] [PubMed] [Google Scholar]
- 104.Wheat T.E., Goldberg E. Sperm-specific lactate dehydrogenase C4: Antigenic structure and immunosuppression of fertility. In: Scandalios J.G., Whitt G.S., editors. Isozymes. Current Topics in Biological and Medical Research. Liss; New York, NY, USA: 1983. pp. 113–130. [PubMed] [Google Scholar]
- 105.Duan C., Goldberg E. Inhibition of lactate dehydrogenase C4 (LDH-C4) blocks capacitation of mouse sperm in vitro. Cytogenet. Genome Res. 2003;103:352–359. doi: 10.1159/000076824. [DOI] [PubMed] [Google Scholar]
- 106.Zimmerman S.W., Yi Y.J., Sutovsky M., van Leeuwen F.W., Conant G., Sutovsky P. Identification and characterization of RING-finger ubiquitin ligase UBR7 in mammalian spermatozoa. Cell. Tissue Res. 2014;356:261–278. doi: 10.1007/s00441-014-1808-x. [DOI] [PubMed] [Google Scholar]
- 107.Zimmerman S.W., Manandhar G., Yi Y.J., Gupta S.K., Sutovsky M., Odhiambo J.F., Powell M.D., Miller D.J., Sutovsky P. Sperm proteasomes degrade sperm receptor on the egg zona pellucida during mammalian fertilization. PLoS ONE. 2011;6:e17256. doi: 10.1371/journal.pone.0017256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jaiswal A., Joshi P., Kumar M.V., Panda J.N., Singh L.N. Angiotensin converting enzyme in the testis and epididymis of mammals. Andrologia. 1984;16:410–416. doi: 10.1111/j.1439-0272.1984.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 109.Yotsumoto H., Sato S., Shibuya M. Localization of angiotensin converting enzyme (dipeptidyl carboxypeptidase) in swine sperm by immunofluorescence. Life Sci. 1984;35:1257–1261. doi: 10.1016/0024-3205(84)90096-1. [DOI] [PubMed] [Google Scholar]
- 110.Krassnigg F., Niederhauser H., Placzek R., Frick J., Schill W.B. Investigations on the functional role of angiotensin converting enzyme (ACE) in human seminal plasma. Adv. Exp. Med. Biol. 1986;198 Pt A:477–485. doi: 10.1007/978-1-4684-5143-6_64. [DOI] [PubMed] [Google Scholar]
- 111.Brentjens J.R., Matsuo S., Andres G.A., Caldwell P.R., Zamboni L. Gametes contain angiotensin converting enzyme (kininase II) Experientia. 1986;42:399–402. doi: 10.1007/BF02118626. [DOI] [PubMed] [Google Scholar]
- 112.Vivet F., Callard P., Gamoudi A. Immunolocalization of angiotensin 1 converting enzyme in the human male genital tract by the avidin-biotin-complex method. Histochemistry. 1987;86:499–502. doi: 10.1007/BF00500623. [DOI] [PubMed] [Google Scholar]
- 113.Dobrinski I., Ignotz G.G., Fagnan M.S., Yudin S.I., Ball B.A. Isolation and characterization of a protein with homology to angiotensin converting enzyme from the periacrosomal plasma membrane of equine spermatozoa. Mol. Reprod. Dev. 1997;48:251–260. doi: 10.1002/(SICI)1098-2795(199710)48:2<251::AID-MRD13>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 114.Gatti J.L., Druart X., Guerin Y., Dacheux F., Dacheux J.L. A 105- to 94-kilodalton protein in the epididymal fluids of domestic mammals is angiotensin I-converting enzyme (ACE); evidence that sperm are the source of this ACE. Biol. Reprod. 1999;60:937–945. doi: 10.1095/biolreprod60.4.937. [DOI] [PubMed] [Google Scholar]
- 115.Reeves P.G., O’Dell B.L. Zinc deficiency in rats and angiotensin-converting enzyme activity: Comparative effects on lung and testis. J. Nutr. 1988;118:622–626. doi: 10.1093/jn/118.5.622. [DOI] [PubMed] [Google Scholar]
- 116.Singh U.S., Kumar M.V., Panda J.N. Angiotensin converting enzyme in semen and its possible role in capacitation. Andrologia. 1985;17:472–475. doi: 10.1111/j.1439-0272.1985.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 117.Kondoh G., Tojo H., Nakatani Y., Komazawa N., Murata C., Yamagata K., Maeda Y., Kinoshita T., Okabe M., Taguchi R., et al. Angiotensin-converting enzyme is a GPI-anchored protein releasing factor crucial for fertilization. Nat. Med. 2005;11:160–166. doi: 10.1038/nm1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zigo M., Jonakova V., Sulc M., Manaskova-Postlerova P. Characterization of sperm surface protein patterns of ejaculated and capacitated boar sperm, with the detection of ZP binding candidates. Int. J. Biol. Macromol. 2013;61:322–328. doi: 10.1016/j.ijbiomac.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 119.Einarsson S., Gustafsson B., Settergren I. Alkaline phosphatase activity of epididymal contents in boars with normal or reduced spermatogenesis. Andrologia. 1976;8:25–28. doi: 10.1111/j.1439-0272.1976.tb01640.x. [DOI] [PubMed] [Google Scholar]
- 120.Bell D.J., Lake P.E. A comparison of phosphomonesterase activities in the seminal plasmas of the domestic cock, turkey tom, boar, bull, buck rabbit and of man. J. Reprod. Fertil. 1962;3:363–368. doi: 10.1530/jrf.0.0030363. [DOI] [PubMed] [Google Scholar]
- 121.Soucek D.A., Vary J.C. Some properties of acid and alkaline phosphates from boar sperm plasma membranes. Biol. Reprod. 1984;31:687–693. doi: 10.1095/biolreprod31.4.687. [DOI] [PubMed] [Google Scholar]
- 122.Bucci D., Isani G., Giaretta E., Spinaci M., Tamanini C., Ferlizza E., Galeati G. Alkaline phosphatase in boar sperm function. Andrology. 2014;2:100–106. doi: 10.1111/j.2047-2927.2013.00159.x. [DOI] [PubMed] [Google Scholar]
- 123.Gillis B.A., Tamblyn T.M. Association of bovine sperm aldolase with sperm subcellular components. Biol. Reprod. 1984;31:25–35. doi: 10.1095/biolreprod31.1.25. [DOI] [PubMed] [Google Scholar]
- 124.Mildvan A.S., Kobes R.D., Rutter W.J. Magnetic resonance studies of the role of the divalent cation in the mechanism of yeast aldolase. Biochemistry. 1971;10:1191–1204. doi: 10.1021/bi00783a016. [DOI] [PubMed] [Google Scholar]
- 125.Dafeldecker W.P., Vallee B.L. Organ-specific human alcohol dehydrogenase: Isolation and characterization of isozymes from testis. Biochem. Biophys. Res. Commun. 1986;134:1056–1063. doi: 10.1016/0006-291X(86)90358-X. [DOI] [PubMed] [Google Scholar]
- 126.Khokha A.M., Voronov P.P., Zimatkin S.M. Immunoenzyme and immunohistochemical analysis of class III alcohol dehydrogenase from human testis. Biokhimiia. 1994;59:997–1002. [PubMed] [Google Scholar]
- 127.Cho C. Testicular and epididymal ADAMs: Expression and function during fertilization. Nat. Rev. Urol. 2012;9:550–560. doi: 10.1038/nrurol.2012.167. [DOI] [PubMed] [Google Scholar]
- 128.Chunghee C. Mammalian ADAMs with Testis-Specific or -Predominant Expression. In: Hooper N., Lendeckel U., editors. The ADAM Family of Proteases. Springer; Dordrecht, The Netherlands: 2005. pp. 239–259. [Google Scholar]
- 129.Loeb J. On Some Non-Specific Factors for the Entrance of the Spermatozoon into the Egg. Science. 1914;40:316–318. doi: 10.1126/science.40.1026.316. [DOI] [PubMed] [Google Scholar]
- 130.Rogers J., Yanagimachi R. Release of hyaluronidase from guinea-pig spermatozoa through an acrosome reaction initiated by calcium. J. Reprod. Fertil. 1975;44:135–138. doi: 10.1530/jrf.0.0440135. [DOI] [PubMed] [Google Scholar]
- 131.Yanagimachi R., Kanoh Y. Manner of Sperm Entry in Herring Egg, with Special Reference to the Role of Calcium Ions in Fertilization. J. Fac. Sci. Hokkaido Univ. 1953;11:487. [Google Scholar]
- 132.Yanagimachi R., Usui N. Calcium dependence of the acrosome reaction and activation of guinea pig spermatozoa. Exp. Cell Res. 1974;89:161–174. doi: 10.1016/0014-4827(74)90199-2. [DOI] [PubMed] [Google Scholar]
- 133.Gervasi M.G., Visconti P.E. Chang’s meaning of capacitation: A molecular perspective. Mol. Reprod. Dev. 2016;83:860–874. doi: 10.1002/mrd.22663. [DOI] [PubMed] [Google Scholar]
- 134.Chang M.C. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–698. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- 135.Kerns K., Zigo M., Drobnis E.Z., Sutovsky M., Sutovsky P. Zinc ion flux during mammalian sperm capacitation. Nat. Commun. 2018;9:2061. doi: 10.1038/s41467-018-04523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.De Jonge C. Biological basis for human capacitation-revisited. Hum. Reprod. Update. 2017;23:289–299. doi: 10.1093/humupd/dmw048. [DOI] [PubMed] [Google Scholar]
- 137.Florman H.M., Jungnickel M.K., Sutton K.A. Shedding light on sperm pHertility. Cell. 2010;140:310–312. doi: 10.1016/j.cell.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 138.Zeng Y., Oberdorf J.A., Florman H.M. pH regulation in mouse sperm: Identification of Na(+)-, Cl(−)-, and HCO3(-)-dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev. Biol. 1996;173:510–520. doi: 10.1006/dbio.1996.0044. [DOI] [PubMed] [Google Scholar]
- 139.Wang D., Hu J., Bobulescu I.A., Quill T.A., McLeroy P., Moe O.W., Garbers D.L. A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC) Proc. Natl. Acad. Sci. USA. 2007;104:9325–9330. doi: 10.1073/pnas.0611296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lishko P.V., Botchkina I.L., Fedorenko A., Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 2010;140:327–337. doi: 10.1016/j.cell.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 141.Henkel R., Bittner J., Weber R., Hüther F., Miska W. Relevance of zinc in human sperm flagella and its relation to motility. Fertil. Steril. 1999;71:1138–1143. doi: 10.1016/S0015-0282(99)00141-7. [DOI] [PubMed] [Google Scholar]
- 142.Wroblewski N., Schill W.-B., Henkel R. Metal chelators change the human sperm motility pattern. Fertil. Steril. 2003;79:1584–1589. doi: 10.1016/S0015-0282(03)00255-3. [DOI] [PubMed] [Google Scholar]
- 143.Miller M.R., Kenny S.J., Mannowetz N., Mansell S.A., Wojcik M., Mendoza S., Zucker R.S., Xu K., Lishko P.V. Asymmetrically Positioned Flagellar Control Units Regulate Human Sperm Rotation. Cell Rep. 2018;24:2606–2613. doi: 10.1016/j.celrep.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kerns K., Morales P., Sutovsky P. Regulation of Sperm Capacitation by the 26S Proteasome: An Emerging New Paradigm in Spermatology. Biol. Reprod. 2016;94:117. doi: 10.1095/biolreprod.115.136622. [DOI] [PubMed] [Google Scholar]
- 145.Yokota N., Sawada H. Sperm proteasomes are responsible for the acrosome reaction and sperm penetration of the vitelline envelope during fertilization of the sea urchin Pseudocentrotus depressus. Dev. Biol. 2007;308:222–231. doi: 10.1016/j.ydbio.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 146.Sánchez R., Deppe M., Schulz M., Bravo P., Villegas J., Morales P., Risopatrón J. Participation of the sperm proteasome during in vitro fertilisation and the acrosome reaction in cattle. Andrologia. 2011;43:114–120. doi: 10.1111/j.1439-0272.2009.01031.x. [DOI] [PubMed] [Google Scholar]
- 147.Chakravarty S., Bansal P., Sutovsky P., Gupta S.K. Role of proteasomal activity in the induction of acrosomal exocytosis in human spermatozoa. Reprod. Biomed. Online. 2008;16:391–400. doi: 10.1016/S1472-6483(10)60601-3. [DOI] [PubMed] [Google Scholar]
- 148.Ambroggio X.I., Rees D.C., Deshaies R.J. JAMM: A Metalloprotease-Like Zinc Site in the Proteasome and Signalosome. PLOS Biol. 2003;2:e2. doi: 10.1371/journal.pbio.0020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim I., Kim C.H., Kim J.H., Lee J., Choi J.J., Chen Z.A., Lee M.G., Chung K.C., Hsu C.Y., Ahn Y.S. Pyrrolidine dithiocarbamate and zinc inhibit proteasome-dependent proteolysis. Exp. Cell Res. 2004;298:229–238. doi: 10.1016/j.yexcr.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 150.Clapper D.L., Davis J.A., Lamothe P.J., Patton C., Epel D. Involvement of zinc in the regulation of pHi, motility, and acrosome reactions in sea urchin sperm. J. Cell Biol. 1985;100:1817–1824. doi: 10.1083/jcb.100.6.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Michailov Y., Ickowicz D., Breitbart H. Zn2+-stimulation of sperm capacitation and of the acrosome reaction is mediated by EGFR activation. Dev. Biol. 2014;396:246–255. doi: 10.1016/j.ydbio.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 152.Guidobaldi H.A., Cubilla M., Moreno A., Molino M.V., Bahamondes L., Giojalas L.C. Sperm chemorepulsion, a supplementary mechanism to regulate fertilization. Hum. Reprod. 2017;32:1560–1573. doi: 10.1093/humrep/dex232. [DOI] [PubMed] [Google Scholar]
- 153.Stephenson J.L., Brackett B.G. Influences of zinc on fertilisation and development of bovine oocytes in vitro. Zygote. 1999;7:195–201. doi: 10.1017/S096719949900057X. [DOI] [PubMed] [Google Scholar]
- 154.Backstrom J.R., Miller C.A., Tokes Z.A. Characterization of neutral proteinases from Alzheimer-affected and control brain specimens: Identification of calcium-dependent metalloproteinases from the hippocampus. J. Neurochem. 1992;58:983–992. doi: 10.1111/j.1471-4159.1992.tb09352.x. [DOI] [PubMed] [Google Scholar]
- 155.Beek J., Nauwynck H., Maes D., Van Soom A. Inhibitors of zinc-dependent metalloproteases hinder sperm passage through the cumulus oophorus during porcine fertilization in vitro. Reproduction. 2012;144:687–697. doi: 10.1530/REP-12-0311. [DOI] [PubMed] [Google Scholar]
- 156.Andreychenko S.V., Klepko A.V., Gorban L.V., Motryna O.A., Grubska L.V., Trofimenko O.V. Post-Chornobyl remote radiation effects on human sperm and seminal plasma characteristics. Exp. Oncol. 2016;38:245–251. [PubMed] [Google Scholar]
- 157.Colagar A.H., Marzony E.T., Chaichi M.J. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr. Res. 2009;29:82–88. doi: 10.1016/j.nutres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 158.Dissanayake D., Wijesinghe P.S., Ratnasooriya W.D., Wimalasena S. Effects of zinc supplementation on sexual behavior of male rats. J. Hum. Reprod. Sci. 2009;2:57–61. doi: 10.4103/0974-1208.57223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mahajan S.K., Prasad A.S., McDonald F.D. Sexual dysfunction in uremic male: Improvement following oral zinc supplementation. Contrib. Nephrol. 1984;38:103–111. doi: 10.1159/000408073. [DOI] [PubMed] [Google Scholar]
- 160.Prasad A.S., Mantzoros C.S., Beck F.W., Hess J.W., Brewer G.J. Zinc status and serum testosterone levels of healthy adults. Nutrition. 1996;12:344–348. doi: 10.1016/S0899-9007(96)80058-X. [DOI] [PubMed] [Google Scholar]
- 161.Kilic M. Effect of fatiguing bicycle exercise on thyroid hormone and testosterone levels in sedentary males supplemented with oral zinc. Neuro Endocrinol. Lett. 2007;28:681–685. [PubMed] [Google Scholar]
- 162.Underwood E., Somers M. Studies of zinc nutrition in sheep. I. The relation of zinc to growth, testicular development, and spermatogenesis in young rams. Aust. J. Agric. Res. 1969;20:889–897. doi: 10.1071/AR9690889. [DOI] [Google Scholar]
- 163.Irani M., Amirian M., Sadeghi R., Lez J.L., Latifnejad Roudsari R. The Effect of Folate and Folate Plus Zinc Supplementation on Endocrine Parameters and Sperm Characteristics in Sub-Fertile Men: A Systematic Review and Meta-Analysis. Urol. J. 2017;14:4069–4078. [PubMed] [Google Scholar]
- 164.Hadwan M.H., Almashhedy L.A., Alsalman A.R. Oral Zinc Supplementation Restores Superoxide Radical Scavengers to Normal Levels in Spermatozoa of Iraqi Asthenospermic Patients. Int. J. Vitam. Nutr. Res. 2015;85:165–173. doi: 10.1024/0300-9831/a000235. [DOI] [PubMed] [Google Scholar]
- 165.Arangasamy A., Venkata Krishnaiah M., Manohar N., Selvaraju S., Guvvala P.R., Soren N.M., Reddy I.J., Roy K.S., Ravindra J.P. Advancement of puberty and enhancement of seminal characteristics by supplementation of trace minerals to bucks. Theriogenology. 2018;110:182–191. doi: 10.1016/j.theriogenology.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 166.Leitzmann M.F., Stampfer M.J., Wu K., Colditz G.A., Willett W.C., Giovannucci E.L. Zinc Supplement Use and Risk of Prostate Cancer. J. Natl. Cancer Inst. 2003;95:1004–1007. doi: 10.1093/jnci/95.13.1004. [DOI] [PubMed] [Google Scholar]
- 167.Plum L.M., Rink L., Haase H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health. 2010;7:1342–1365. doi: 10.3390/ijerph7041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.O’Flaherty C., Fournier D.M. Reactive oxygen species and protein modifications in spermatozoa. Biol. Reprod. 2017;97:577–585. doi: 10.1093/biolre/iox104. [DOI] [PubMed] [Google Scholar]
- 169.Powell S.R. The antioxidant properties of zinc. J. Nutr. 2000;130:1447S–1454S. doi: 10.1093/jn/130.5.1447S. [DOI] [PubMed] [Google Scholar]
- 170.Talevi R., Barbato V., Fiorentino I., Braun S., Longobardi S., Gualtieri R. Protective effects of in vitro treatment with zinc, d-aspartate and coenzyme q10 on human sperm motility, lipid peroxidation and DNA fragmentation. Reprod. Biol. Endocrinol. 2013;11:1–10. doi: 10.1186/1477-7827-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gualtieri R., Barbato V., Fiorentino I., Braun S., Rizos D., Longobardi S., Talevi R. Treatment with zinc, d-aspartate, and coenzyme Q10 protects bull sperm against damage and improves their ability to support embryo development. Theriogenology. 2014;82:592–598. doi: 10.1016/j.theriogenology.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 172.Posthuma L., Baerselman R., Van Veen R.P.M., Dirven-Van Breemen E.M. Single and Joint Toxic Effects of Copper and Zinc on Reproduction ofEnchytraeus crypticusin Relation to Sorption of Metals in Soils. Ecotoxicol. Environ. Saf. 1997;38:108–121. doi: 10.1006/eesa.1997.1568. [DOI] [PubMed] [Google Scholar]
- 173.Barceloux D.G., Barceloux D. Zinc. J. Toxicol. Clin. Toxicol. 1999;37:279–292. doi: 10.1081/CLT-100102426. [DOI] [PubMed] [Google Scholar]
- 174.Johnson F.O., Gilbreath E.T., Ogden L., Graham T.C., Gorham S. Reproductive and developmental toxicities of zinc supplemented rats. Reprod. Toxicol. 2011;31:134–143. doi: 10.1016/j.reprotox.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 175.Manzo S., Schiavo S., Oliviero M., Toscano A., Ciaravolo M., Cirino P. Immune and reproductive system impairment in adult sea urchin exposed to nanosized ZnO via food. Sci. Total Environ. 2017;599–600:9–13. doi: 10.1016/j.scitotenv.2017.04.173. [DOI] [PubMed] [Google Scholar]
- 176.Merrells K.J., Blewett H., Jamieson J.A., Taylor C.G., Suh M. Relationship between abnormal sperm morphology induced by dietary zinc deficiency and lipid composition in testes of growing rats. Br. J. Nutr. 2009;102:226–232. doi: 10.1017/S0007114508159037. [DOI] [PubMed] [Google Scholar]
- 177.Ding B., Zhong Q. Zinc deficiency: An unexpected trigger for autophagy. J. Biol. Chem. 2017;292:8531–8532. doi: 10.1074/jbc.H116.762948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jaiswal B.S., Eisenbach M. Capacitation. In: Hardy D.M., editor. Fertilization. Academic Press; Cambridge, MA, USA: 2002. pp. 57–117. [Google Scholar]
- 179.Aitken R.J., Baker M.A., Nixon B. Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress? Asian J. Androl. 2015;17:633–639. doi: 10.4103/1008-682X.153850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yi Y.J., Manandhar G., Oko R.J., Breed W.G., Sutovsky P. Mechanism of sperm-zona pellucida penetration during mammalian fertilization: 26S proteasome as a candidate egg coat lysin. Soc. Reprod. Fertil. Suppl. 2007;63:385–408. [PubMed] [Google Scholar]