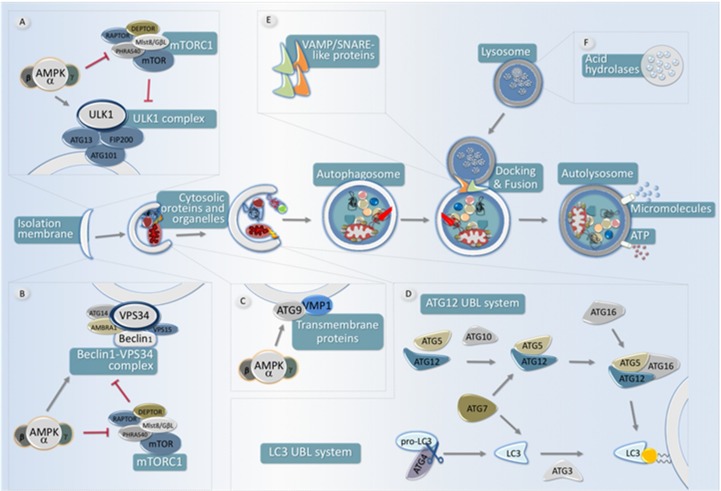
Figure 3.
Scheme of the main steps for autophagy and their regulation by AMPK. An autophagy pathway starts with the formation of the isolation membrane, also known as phagophore. The autophagy implicates the coordinated temporal and spatial activation of numerous molecular components. (A): The ULK1-FIP200-ATG13-ATG101 complex is responsible for initiating the autophagic process. The activity of this protein complex is antagonistically regulated by mTORC1 (inhibitory phosphorylation) and by AMPK, which both activates the ULK1 complex as well as inhibits the activity of the mTORC1 complex. (B): The ClassIII PI3K complex formed by VPS34, Beclin1, ATG14, AMBRA1, and other subunits creates a membrane domain enriched in PtsIns3P, which drives the nucleation of ATG (AuTophaGy-related) proteins in the phagophore, either directly or indirectly. AMPK is able to increase the pro-autophagic function of this complex and to enhance its formation, whereas mTORC1 activity negatively regulates its function. (C): Two different transmembrane proteins, the vacuole membrane protein 1 (VMP1) and ATG9 participate in the recruitment of membranes to the phagophore. AMPK is able to phosphorylate ATG9, which increases its recruitment towards autophagosome formation sites. (D): Two ubiquitin-like (UBL) protein conjugation systems (ATG12- and LC3- UBLs) involving the participation of ATG4 cysteine proteinases (which activate LC3 by cleaving its carboxyl terminus), the E1-like enzyme ATG7 (common to both conjugation systems), and the E2-like enzymes ATG10 (ATG12 system), and ATG3 (LC3 system). In coordination, the activity of both systems is required to conjugate LC3 (and other members of this protein family homologous to yeast ATG8) to a phosphatidyl-ethanolamine lipid at the nascent pre-autophagosomal membrane. (E): Upon completion, fully-formed autophagosomes move along the microtubule network, eventually fusing with a lysosome, thus acquiring hydrolytic activity, and thus becoming autolysosomes. Several SNARE-like proteins (i.e., Syntaxin17 and VAMP8, among others) are required for efficient fusion between lysosomes and autophagosomes. Once content and inner membrane are degraded by acidic hydrolases, the resultant molecules (amino acids, nucleotides, lipids, etc.) are recycled back to the cytoplasm by membrane permeases.