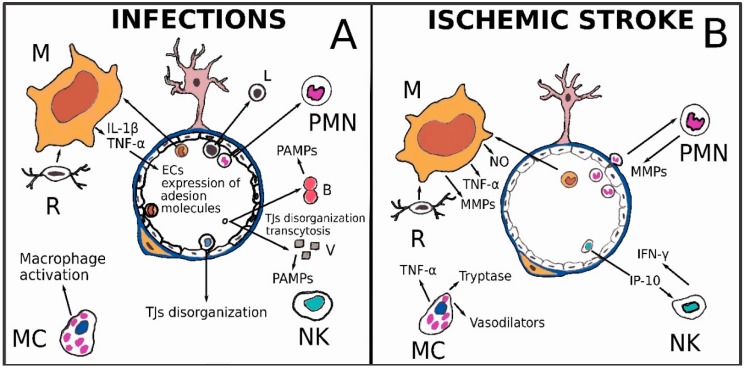
Figure 2.
(A) Infectious agents can destroy or displace TJs’ constituent proteins or cross the BBB, also through the initiation of caveolin-dependent endocytosis mechanisms. Once pathogens have entered the nervous system, they are able to stimulate innate immunity cells through their PRRs. Cytokines such as tumor necrosis factor-α and interleukin-1β are secreted in the inflammatory environment and stimulate ECs belonging to the BBB to upregulate cell adhesion molecules like VCAM-1, ICAM-1 and E-selectin, allowing new immune cells to enter the nervous tissue. Other immune cells, such as lymphocytes and NK cells, can disarrange the TJs’ structure and increase vessel permeability. Mast cells releasing mediators such as histamine, tryptase or CCL5, represents another possible way of macrophage activation; (B) Microglia (R) is rapidly activated after the ischemic event and releases mediators such as TNF-α, superoxide anion and NO, able to recruit circulating monocytes. Microglia and macrophages (M) show a pro-inflammatory activation pattern and produce molecules involved in oxidative damage and metalloproteinases (MMPs) that cause BBB leakage. After prolonged ischemia, Polymorphonuclear Neutrophils (PMNs) can be found in infarcted parenchyma. PMNs share with macrophages and microglia the ability to produce various molecules, such as MMPs, detrimental for BBB. Mast cells (MCs) degranulate after stroke and secrete compounds capable of increasing vascular permeability. NK cells are active during the acute phase of ischemic stroke. NK cells, in cerebral ischemia, promote neural cells necrosis and damage of BBB via IFN-γ. Moreover, cytokines such as IP-10 secreted by CXCR3-activated NK cells, (a chemokine of the CXC subfamily) intensify injury to the BBB. Indeed, IP-10 expression (detectable in ischemic brain tissue) may induce NK cell infiltration following cerebral ischemia.