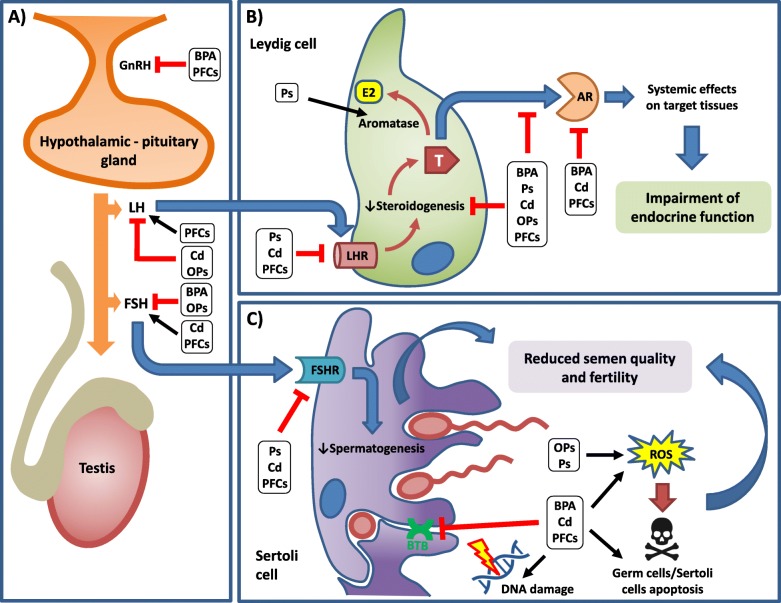
Fig. 1.
Schematic representation of endocrine disruptors’ (EDs) effects on male fertility and the related mechanisms of toxicity. Results from both pre-clinical and clinical studies are summarized for each ED. If proposed effects are common to more EDs, they are reported together within black squares. Black arrows refer to stimulatory pathways. Red arrows with blunt ends represent inhibitory regulation. Hypotalamic-pituitary regulation of testicular function is impaired by most EDs (a). Within the testis, gonadotropins stimulates steroidogenesis in Leydig cells (b) and spermatogenesis in Sertoli cells (c). Overall, EDs disrupt endocrine function by reducing testosterone release or its activity on target tissues. In addition, EDs can reduce semen quality by directly impairing cell structure/viability or indirectly by interfering with hormonal patwhays. GnRH: Gonadotropin-releasing hormone; LH: Lutehinizing Hormone; FSH: Follicle-Stimulating Hormone; T: testosterone; AR: Androgen Receptor; FSHR: FSH Receptor; LHR: LH Receptor; E2: Estradiol; ROS: Reactive Oxygen Species; BTB: Blood-Testis Barrier; BPA: Bisphenol A; Ps: Phtalathes; Cd: Cadmium; Ops: Organophosphate pesticides; PFCs: Perfluoroalkyl Compounds