Abstract
Malaria remains a significant cause of morbidity and mortality worldwide, particularly in infants and children. Some studies have reported that exposure to malaria antigens in utero results in the development of tolerance, which could contribute to poor immunity to malaria in early life. However, the effector T cell response to pathogen-derived antigens encountered in utero, including malaria, has not been well characterized. Here, we assessed the frequency, phenotype, and function of cord blood T cells from Ugandan infants born to mothers with and without placental malaria. We found that infants born to mothers with active placental malaria had elevated frequencies of proliferating effector memory fetal CD4+ T cells and higher frequencies of CD4+ and CD8+ T cells that produced inflammatory cytokines. Fetal CD4+ and CD8+ T cells from placental malaria-exposed infants exhibited greater in vitro proliferation to malaria antigens. Malaria-specific CD4+ T cell proliferation correlated with prospective protection from malaria during childhood. These data demonstrate that placental malaria is associated with the generation of proinflammatory malaria-responsive fetal T cells. These findings add to our current understanding of fetal immunity and indicate that a functional and protective pathogen-specific T cell response can be generated in utero.
INTRODUCTION
Pregnancy-associated malaria, including placental malaria (PM), remains a significant global health threat, resulting in low birth weight, preterm birth, and other complications that contribute to an estimated 100,000 deaths per year (1, 2). PM is characterized by the accumulation of parasite-infected erythrocytes in the placenta, accompanied by pathological changes, including the presence of hemozoin, infiltration of monocytes and macrophages, and deposition of perivillous fibrin (3). Although true congenital malaria infection is rare, malaria antigens are known to cross the placental barrier and enter fetal circulation (4, 5). Some studies have reported that the cord blood of infants born to women with PM contains increased frequencies of regulatory T cells (Tregs) that could suppress malaria-specific T cell responses (6–11), whereas other groups have reported no association of Tregs with PM (12, 13). Thus, it remains unclear precisely how the fetal immune system responds to in utero malaria exposure and whether this exposure has consequences for antimalarial immunity during childhood.
The fetus is predisposed toward the induction of tolerance upon encounter with foreign antigens (14). At birth, cord blood fetal T cells are primarily naïve in phenotype (CD45RA+) and fetal CD4+ T cells generally exhibit a differentiation bias away from T helper 1 (TH1) cytokine production and toward TH2 and Treg functions (14–18). However, recent work has demonstrated that even during healthy pregnancy, in the absence of known exposure to an intrauterine pathogen, human cord blood contains effector memory CD4+ T cells (19). Although in utero exposure to some viruses, including hepatitis B virus (20), hepatitis C virus (21), HIV (22–24), and cytomegalovirus (CMV) (25, 26), has been reported to result in priming of T cells before birth, there has been limited characterization of their phenotype, function, and antigen specificity. Moreover, protection against infection during infancy by T cells primed in utero has not been demonstrated.
To investigate the impact of in utero malaria exposure on fetal T cell immunity, we assessed CD4+ and CD8+ T cell frequency, phenotype, and function in the cord blood of a cohort of182 infants born to mothers with and without PM, acquired as part of a study of pregnant women and infants in a highly malaria-endemic region of Uganda (27). Our findings indicate that the human fetus is capable of mounting functional effector T cell responses in utero and suggest that these responses may afford protection against pathogen exposure in early childhood.
RESULTS
Fetal effector memory T cells in cord blood of PM-exposed infants
To better understand how PM alters the fetal T cell compartment, we used cord blood samples from a subset of infants enrolled in a clinical trial of prenatal and early childhood malaria intermittent preventative treatment (IPT) in malaria-endemic Uganda. In this study, HIV-negative pregnant women were enrolled between 12 and 20 weeks of gestation and randomized to receive standard versus enhanced malaria IPT during pregnancy (27). Women were evaluated monthly for malaria parasitemia from enrollment until delivery, and women presenting with fever or other symptoms of malaria were evaluated by blood smear. At birth, cord blood and placental tissue were assessed for the presence of malaria parasite DNA by loop-mediated isothermal amplification (LAMP) and for histopathologic evidence of PM, as previously described (27–29). All infants in the clinical trial were included in this analysis if they had cord blood mononuclear cells (CBMCs) collected in sufficient quantity and were CMV negative at birth (fig. S1 and table S1).
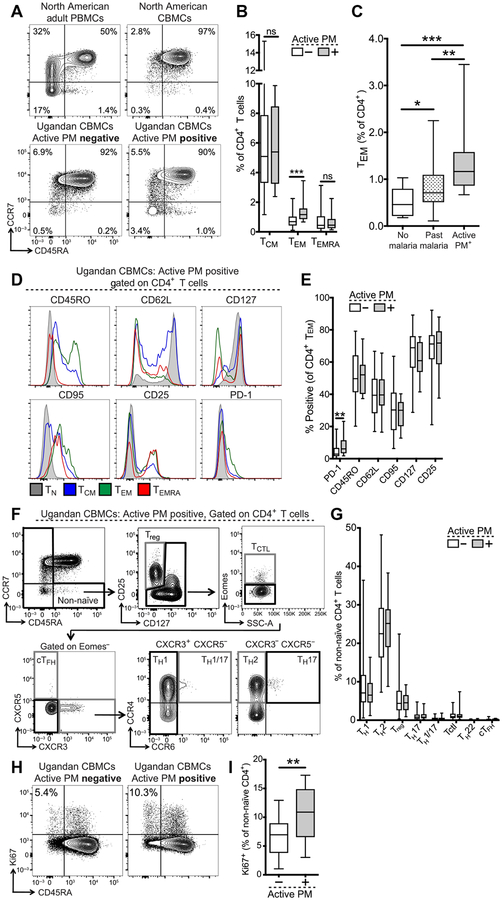
We compared the frequency and phenotype of effector and memory CD4+ and CD8+ T cells in the cord blood of infants born to mothers with and without active PM, as defined by the presence or absence of malaria parasite DNA in the placenta. As flow cytometric staining controls, North American healthy adult PBMCs (peripheral blood mononuclear cells) and CBMCs were assessed in parallel. On the basis of the expression of CCR7 and CD45RA, we identified populations of central memory (TCM), effector memory (TEM), and effector memory RA+ (TEMRA) CD4+ and CD8+ T cells in cord blood (Fig. 1A). We observed a significantly higher frequency of CD4+ TEM cells in infants born to mothers with active PM compared to those born to mothers without active PM (P = 0.0001; Fig. 1B). The subset of these infants whose placentas had parasites visualized by histo-pathology also had significantly higher frequencies of CD4+ TEM cells than those without parasites (P = 0.018; fig. S2A). We further stratified mothers without active PM into those with no evidence of malaria infection during pregnancy and those with past malaria infection (based on detection of malaria parasite DNA in maternal peripheral blood during pregnancy or positive placental histopathology, but no malaria parasite DNA in the placenta). The frequency of CD4+ TEM cells among infants born to mothers with past malaria was higher than in those with no evidence of infection but was lower than in those with active PM (Fig. 1C). CD4+ TEM frequencies did not differ by maternal gravidity or IPT randomization arm (fig. S2, B and C). Effector memory differentiation was also observed among cord blood CD8+ T cells; however, the frequency of CD8+ TCM, TEM, and TEMRA cells did not differ between exposed and unexposed infants (fig. S3, A and B). To confirm that the activated T cell populations seen in PM-exposed infants were fetally derived, as opposed to cells of maternal origin (30, 31), we performed fluorescence in situ hybridization to detect X and Y chromosomes in sorted non-naïve CD4+ T cells from male infant cord blood. We detected exclusively fetal T cells (that is, XY) in these samples (fig. S4, A and B), confirming their fetal origin.
Fig. 1. Fetal effector memory CD4+ T cell differentiation in cord blood of malaria-exposed infants.
(A) Representative flow plots of CD4+ T cell effector memory subsets. TCM, CD45RA−CCR7+; TEM, CD45RA−CCR7−; and TEMRA, CD45RA+CCR7−. Values indicate % of CD4+ T cells. (B) Quantification of CD4+ T cell subsets in infants born to mothers with (gray bar; n = 14) or without (white bar; n = 55) active PM. (C) Frequency of TEM cells in infants born to mothers without malaria during pregnancy (white bar; n = 10), with past malaria infection (striped bar; n = 45), or with active PM (gray bar; n = 14). ns, not significant. (D) Representative histograms of CD4+ T cell subset phenotype from a representative PM-exposed infant. TCTL, cytotoxic T lymphocyte; cTFH, circulating T follicular helper cell. (E) Quantification of % positive for phenotypic markers on CD4+ TEM cells in active PM-exposed (gray bar; n = 14) versus unexposed (white bar; n = 55) infants. (F) Gating strategy for CD4+ TH subsets; representative of an active PM-exposed infant. (G) Quantification of CD4+ TH subsets (% of non-naïve CD4+ T cells) in infants born to mothers with (gray bar; n = 20) versus without (white bar; n = 154) active PM. (H) Representative flow plots of Ki67 and CD45RA expression. Values indicate % Ki67+ cells of non-naïve CD4+ T cells (CD45RA−). (I) Quantification of non-naïve Ki67+ T cells in active PM-exposed (gray bar; n = 11) versus unexposed (white bar; n = 57) infants. All representative flow plots were gated on live CD14−CD19−γδTCR−CD3+CD8−CD4+ T cells. *P < 0.05, **P < 0.01, ***P < 0.001, Kruskal-Wallis and Wilcoxon rank sum test.
Given the elevated frequencies of CD4+ TEM cells in infants born to mothers with active PM, we further characterized the activation profile, differentiation status, and TH subset distribution of fetal non-naïve CD4+ and CD8+ T cells (TEM, TCM, and TEMRA) directly ex vivo. Compared to naïve T cells (TN), CD4+ TEM cells were CD45ROhighCD62LlowCD127low and expressed the proapoptotic marker CD95/Fas, the high-affinity interleukin-2 (IL-2) receptor CD25, and the inhibitory receptor programmed cell death-1 (PD-1) (Fig. 1D). CD4+ TEM cells from active PM-exposed infants expressed significantly more PD-1 than did those from unexposed infants (P = 0.006), but there were no differences in other activation markers (Fig. 1E). We also used a combination of activation marker, chemokine receptor, and transcription factor expression to identify eight distinct TH subsets (32) within non-naïve CD4+ T cells (Fig. 1F). We found this compartment to consist predominantly of TH1-, TH2-, and Treg-phenotype CD4+ T cells. The frequency of CD4+ TH subsets did not differ between infants born to mothers with or without active PM (Fig. 1G). Heterogeneity in the non-naïve CD4+ T cell compartment was also evident at the transcriptional level, with coexpression of multiple transcription factors associated with TH1 (Prdml, Tbx21, and BATF), TH2 (Gata3), and TH17 (RoRγT) cells (fig. S5), again with no difference observed between active PM-exposed and unexposed infants. However, expression of Ki67 by non-naïve CD4+ T cells was significantly higher in infants born to mothers with active PM (P < 0.0001), suggesting greater in vivo proliferation of CD4+ T cells (Fig. 1, H and I). We found no significant difference in the activation status (fig. S3B) or Ki67 expression (fig. S3C) of non-naïve CD8+ T cells. However, we did observe an increased frequency of CXCR3+ Tbet+ Eomes+ non-naïve CD8+ T cells among infants born to mothers with active PM (fig. S3D), suggesting some effector differentiation of the CD8+ T cell compartment. Thus, we have identified a population of fetal effector memory CD4+ T cells that is enriched in the cord blood of infants born to mothers with active PM and exhibits evidence of in vivo activation and proliferation.
Inflammatory cytokine production by cord blood CD4+ and CD8+ T cells from PM-exposed infants
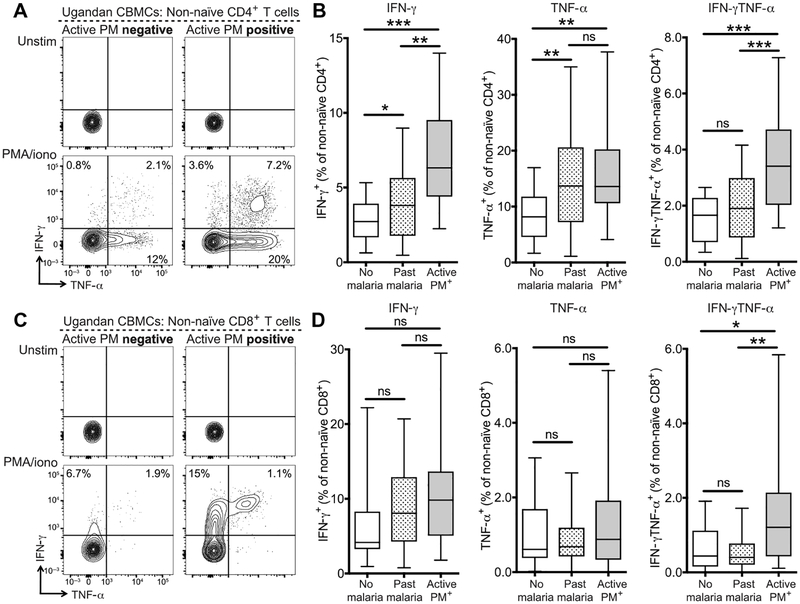
We next evaluated the functional capabilities of cord blood CD4+ and CD8+ T cells in PM-exposed infants by assessing cytokine production by intracellular cytokine staining (ICS) after in vitro stimulation with phorbol 12-myristate 13-acetate (PMA)/ionomycin. Infants born to mothers with active PM had significantly higher frequencies of non-naïve CD4+ T cells producing the proinflammatory cytokine interferon-γ (IFN-γ) compared to infants born to mothers with past infection (P = 0.002) or infants born to mothers with no malaria infection during pregnancy (P < 0.001). Similar results were observed for production of tumor necrosis factor-α (TNF-α) and coproduction of IFN-γ and TNF-α (Fig. 2, A and B). Production of IL-2 and IL-8 by CD4+ T cells was also observed but did not differ between exposure groups (fig. S6A), whereas IL-17A and IL-10 were not detected. Similarly, in the CD8+ T cell compartment, active PM-exposed infants had higher frequencies of non-naïve CD8+ T cells producing IFN-γ (P = 0.05) and significantly higher frequencies of non-naïve CD8+ T cells co-producing IFN-γ and TNF-α (no malaria: P = 0.03/past infection: P = 0.005) (Fig. 2, C and D, and fig. S6B). Increased cytokine production by T cells from infants born to mothers with active PM is consistent with the higher frequency of phenotypically activated fetal TEM cells. Together, these findings indicate that in utero exposure to active PM alters both the phenotype and function of the fetal CD4+ and CD8+ T cell compartments by inducing the differentiation of fetal effector T cells, suggesting either an antigen-specific response to malaria or nonspecific bystander activation.
Fig. 2. Inflammatory cytokine production by cord blood CD4+ and CD8+ T cells from PM-exposed infants.
(A) Representative flow plots of ICS for production of IFN-γ and TNF-α by non-naïve CD4+ T cells after 5-hour in vitro stimulation of CBMCs with medium alone (upper) or PMA/ionomycin (lower). Gated on live CD14−CD19−γδTCR−CD3+CD8−CD4+CD45RA− T cells. Values indicate the frequency of cytokine-positive cells within the non-naïve population. (B) Quantification of cytokine-producing cells in infants born to mothers without malaria during pregnancy (white bar; n = 16), with past malaria infection (striped bar; n = 83), or with active PM (gray bar; n = 21). (C) Representative flow plots of ICS for production of IFN-γ and TNF-a by non-naïve CD8+ T cells after 5-hour in vitro stimulation of CBMCs with medium alone (upper) or PMA/ionomycin (lower). Gated on live CD14−CD19−γδTCR−CD3+CD4−CD8+CD45RA− T cells. Values indicate the frequency of cytokine-positive cells within the non-naïve population. (D) Quantification of cytokine-producing cells in infants born to mothers without malaria during pregnancy (white bar; n = 16), with past malaria infection (striped bar; n = 83), or with active PM (gray bar; n = 21). Values calculated by background subtraction of medium-alone controls. *P < 0.05, **P < 0.01, ***P < 0.001, Kruskal-Wallis and Wilcoxon rank sum test.
Proliferation of cord blood CD4+ and CD8+ T cells in response to malaria antigens
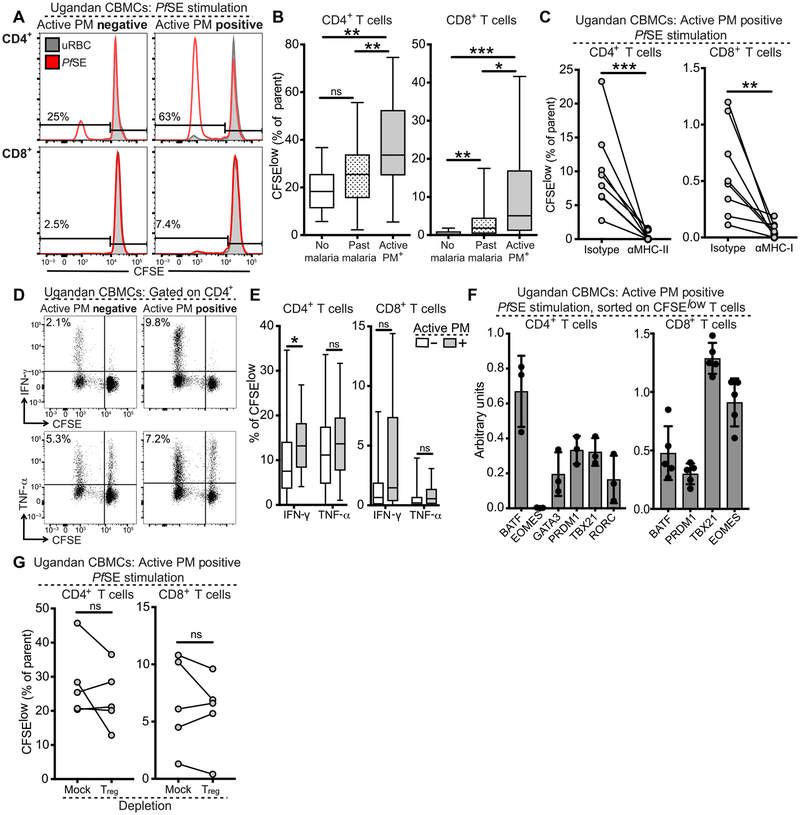
Given the increased frequency of cytokine-producing CD4+ and CD8+ T cells in PM-exposed infants, we next asked whether these cells were specifically responsive to malaria antigens in vitro. To test this, CBMCs were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) and stimulated with Plasmodium falciparum schizont extract (PfSE) or uninfected red blood cells (uRBCs) for 6 days. Proliferating CD4+ T cells (CFSElow, having undergone at least one division) were identifiable in most cord blood samples, whereas CFSElow CD8+ T cells were much rarer (Fig. 3A). We found that infants born to mothers with active PM had significantly higher frequencies of CFSElow CD4+ T cells compared to infants born to mothers with past infection (P = 0.002) or infants born to mothers with no malaria infection during pregnancy (P = 0.002; Fig. 3B). Similarly, compared to infants born to mothers with no malaria infection, CFSElow CD8+ T cell frequencies were increased in infants born to mothers with both past infection (P = 0.004) and active PM (P < 0.001; Fig. 3B). The proliferative T cell response was major histocompatibility complex (MHC) class restricted, as blockade of MHC-I or MHC-II abrogated CD4+ or CD8+ T cell proliferation, respectively (Fig. 3C). To assess whether these malaria-responsive T cells were capable of cytokine production, we restimulated CBMC cultures on day 6 with PMA/ionomycin and evaluated the production of proinflammatory cytokines by ICS. We found that CFSElow proliferating CD4+ and CD8+ T cells produced IFN-γ, TNF-α, and IL-2 (Fig. 3, D and E, and fig. S7A), but no substantial production of IL-17A or IL-8 was detected. Cord blood from infants exposed to active PM contained higher frequencies of CFSElow IFN-γ-producing CD4+ (P = 0.03) and CD8+ (P = 0.05) T cells (Fig. 3E), indicating that active PM induces the differentiation of malaria-responsive T cells in cord blood that are capable of proinflammatory cytokine production. The inflammatory bias of malaria-responsive CD4+ and CD8+ T cells is further supported by the increased gene expression of TH1-associated transcription factors in sorted CFSElow T cells, including TBX21/Tbet, BATF/BATF, and PRDM1/Blimp-1 in CD4+ T cells and TBX21/Tbet, BATF/BATF, PRDM1/Blimp-1, and EOMES/Eomes in CD8+ T cells (Fig. 3F).
Fig. 3. Proliferation of cord blood CD4+ and CD8+ T cells in response to malaria antigens.
(A) Representative CFSE dilution profiles for CD4+ (upper) and CD8+ (lower) T cells after 6-day PfSE (red histogram) or uRBC (gray histogram) stimulation. Values indicate frequency of CFSElow T cells (% of CD4+ or CD8+ T cells). (B) Quantification of CFSElow CD4+ (left) or CD8+ (right) T cells in infants born to mothers without malaria during pregnancy (white bar; n = 11), with past malaria infection (striped bar; n = 69), or with active PM (gray bar; n = 18). (C) CFSElow CD4+ (left) and CD8+ (right) T cell frequencies after 6 days of PfSE stimulation in the presence or absence of αMHC-II or αMHC-I (n = 8). Data are representative of three independent experiments. (D) ICS for cytokine production by CFSE-labeled PfSE-stimulated CBMCs restimulated on day 6 with PMA/ionomycin in infants born to mothers with (gray bar) or without (white bar) active PM. (E) Quantification of CFSElow cytokine-producing CD4+ and CD8+ T cells stratified by infant malaria exposure (active PM−, white bars, n = 79; active PM+, gray bars, n = 17). (F) Transcription factor mRNA expression in sorted CFSElow CD4+ (left) and CD8+ T cells (right) from infants born to active PM+ mothers (n = 3 to 5). Data are representative of three independent experiments. (G) CFSElow CD4+ T cell frequencies in the presence or absence of Tregs. Data are representative of three independent experiments. All representative flow plots were gated on live CD14−CD19−γδTCR−CD3+ T cells. CFSElow frequencies were calculated by background subtraction of uRBC-stimulated and medium-alone controls. *P < 0.05, **P < 0.01, ***P < 0.001, significance assessed by Kruskal-Wallis and Wilcoxon rank sum test for all experiments except MHC blockade and Treg depletion, which were assessed by Wilcoxon matched-pairs signed-rank test.
Next, because several previous studies have reported that in utero exposure to malaria is associated with the induction of Tregs that may suppress fetal T cell activation and proliferation (6–11), we investigated whether depletion of Tregs would influence CD4+ or CD8+ T cell proliferation in response to malaria antigens. We stimulated CFSE-labeled CBMCs with or without CD25+CD127− Tregs by FACS (fluorescence-activated cell sorting) and assessed proliferation in response to PfSE, as above. We found no significant difference in proliferation of CD4+ or CD8+ T cells after depletion of Tregs (Fig. 3G), suggesting that Treg suppression does not substantially affect proliferation of malaria-responsive T cells in this setting. Furthermore, because the Vδ2 subset of γδ T cells has been shown to have intrinsic reactivity to malaria antigens and is expanded in malaria-exposed neonates (33, 34), we similarly assessed whether Vδ2 cell depletion would affect proliferation in response to malaria schizont extract. We found no significant difference in CFSE dilution after depletion of Vδ2 cells (fig. S7B), indicating that bystander cytokine production by Vδ2 T cells is not responsible for the proliferation of fetal T cells in response to malaria antigens.
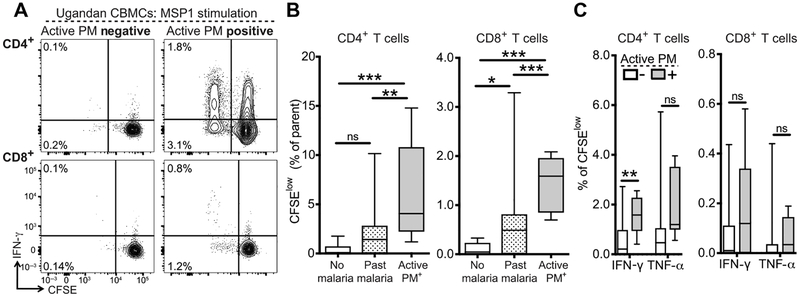
To further confirm the malaria specificity of fetal T cells expanded in response to in utero malaria exposure, we stimulated CFSE-labeled CBMCs with pooled overlapping peptides spanning merozoite surface protein 1 (MSP1), an abundant blood-stage plasmodial protein that is an immunodominant target of malaria-specific T cells (35). After a 6-day stimulation with MSP1 peptides, we observed proliferation of both CD4+ and CD8+ T cells, albeit at much lower frequencies than were observed with PfSE stimulation (Fig. 4A). MSP1 peptide stimulation resulted in significantly higher frequencies of CFSElow CD4+ and CD8+ T cells from infants born to mothers with active PM compared to mothers with past infection (CD4+, P = 0.002; CD8+, P = 0.001) and mothers with no malaria infection during pregnancy (CD4+, P < 0.001; CD8+, P = 0.0002) (Fig. 4B), similar to what we observed after PfSE stimulation. Furthermore, proliferating CD4+ T cells (CFSElow) in active PM-exposed infants produced more IFN-γ after restimulation with PMA/ionomycin (P = 0.001); however, this was not observed for the CD8+ T cell population (Fig. 4C). Together, our results suggest that infants exposed to malaria in utero generate both CD4+ and CD8+ T cells that are responsive to malaria antigens, are MHC restricted, and are capable of proliferation and production of inflammatory cytokines upon reencounter with antigen.
Fig. 4. Proliferation of cord blood CD4+ and CD8+ T cells in response to MSP1 peptide stimulation.
(A) Representative CFSE dilution profiles for CD4+ (upper) and CD8+ (lower) T cells stimulated with MSP1 peptide pools for 6 days and restimulated with PMA/ionomycin. Gated on live CD14−CD19−γδTCR−CD3+ T cells. Values indicate frequency of CFSElow T cells as % CD4+ or CD8+ T cells. (B) Frequency of CFSElow CD4+ (left) or CD8+ (right) T cells in infants born to mothers without malaria during pregnancy (white bar; n = 4), with past malaria infection (striped bar; n = 38), or with active PM (gray bar; n = 9). (C) Quantification of CFSElow cytokine-producing CD4+ and CD8+ T cells stratified by infant malaria exposure (PM−, white bars, n = 41; PM+, gray bars, n = 8). For all experiments, values are calculated by background subtraction of dimethyl sulfoxide-stimulated and medium-alone controls. *P < 0.05, **P < 0.01, ***P < 0.001, Kruskal-Wallis and Wilcoxon rank sum test.
Association between CD4+ T cell proliferation in response to malaria antigens at birth and protection from malaria in infancy
In light of the evidence that malaria-responsive CD4+ T cells capable of effector functions can be primed in utero, we investigated whether these responses were associated with protection against plasmodial infection and symptomatic malaria during infancy and early childhood. Children were followed prospectively until 24 months of age with monthly screening for peripheral parasitemia (by LAMP), as well as monitoring and treatment for symptomatic malaria. All children included in this analysis received IPT with dihydroartemisinin-piperaquine (DP) every 12 weeks (fig. S1). We assessed prospective protection using three separate outcome parameters (clinical malaria, time to first episode of malaria, and parasitemia prevalence). To best assess the relationship between malaria-responsive CD4+ T cells and prospective protection from malaria (which we hypothesized would be nonlinear), we categorized infants into three parsimonious groups based on the percentage of proliferating CD4+ T cells (CFSElow) after in vitro stimulation with PfSE: “low” (<15% CFSElow CD4+ T cells; n = 11), “intermediate” (15 to 30% CFSElow CD4+ T cells; n = 35), and “high” (≥30% CFSElow CD4+ T cells; n = 27). Infants with high proliferation had a >70% lower incidence of clinical malaria in the first 2 years of life compared to infants with low proliferation [incidence rate ratio (IRR), 0.28; P = 0.02; Table 1]. Furthermore, infants with intermediate and high malaria-specific CD4+ T cell proliferation had a reduced time to first malaria episode after birth than children in the low group [intermediate group: hazard ratio (HR), 0.40; P = 0.05; high group: HR, 0.28; P = 0.01; Table 1]. For each 1% increase in CFSElow CD4+ T cells, there was a 4% reduction in the hazard of malaria [HR, 0.96; 95% confidence interval (CI), 0.93 to 0.99; P = 0.025]. Finally, when assessing the risk of parasitemia based on active monthly surveillance from 0 to 24 months of life, infants with high malaria-specific CD4+ T cell proliferation had significantly less parasitemia than children in the other strata [prevalence rate ratio (PRR), 0.33; P = 0.03; Table 1]. In all three of these analyses, the association between malaria-specific CD4+ T cell pro-liferation and protection remained statistically significant after adjustment for maternal malaria during pregnancy (table S1). Together, these results suggest that a higher frequency of cord blood CD4+ T cells that proliferate in response to malaria antigens in vitro (CFSElow CD4+ T cells) results in protection from both parasite infection and symptomatic malaria during the first 2 years of life.
Table 1. CD4+ T cell proliferation in response to malaria antigens associated with protection from childhood malaria.
PY, person years of observation.
| Incidence of clinical malaria through 24 months of age | ||||||
| % PfSE-specific CFSElow CD4+ T cells | n | Episodes | PY | Incidence | IRR (95% CI) | P value |
| Low (<15%) | 11 | 16 | 21.0 | 0.76 | Reference | |
| Intermediate (15 to 30%) | 35 | 36 | 62.5 | 0.58 | 0.79 (0.30–2.06) | 0.63 |
| High (≥30%) | 27 | 10 | 48.3 | 0.21 | 0.28 (0.09–0.84) | 0.02 |
| Time to first episode of malaria after birth | ||||||
| n | Cumulative risk (95% CI) | HR (95% CI) | P value | |||
| Low (<15%) | 11 | 79.6% (51.8–96.8%) | Reference | |||
| Intermediate (15to30%) | 35 | 41.1% (26.3–60.1%) | 0.40 (0.16–0.98) | 0.05 | ||
| High (≥30%) | 27 | 33.8% (18.5–56.5%) | 0.28 (0.11–0.77) | 0.01 | ||
| Detection of malaria parasites at routine monthly visits between 2 and 24 months of age* | ||||||
| n/N | % | PRR (95% CI) | P value | |||
| Low (<15%) | 25/269 | 9.29% | Reference | |||
| Intermediate (15 to 30%) | 56/797 | 7.03% | 0.80 (0.33–1.93) | 0.63 | ||
| High (≥30%) | 19/620 | 3.06% | 0.33 (0.13–0.89) | 0.03 | ||
By blood smear and/or LAMP.
DISCUSSION
Upon encounter with non-self antigens, naïve fetal CD4+ T cells have been reported to preferentially differentiate into Tregs (14), presumably due to the need for reciprocal tolerance between the mother and fetus. T cell-intrinsic mechanisms (18, 36) [for example, hypermethylation of IFNG locus (17)] and T cell-extrinsic mechanisms [for example, differing antigen presenting cell function (37–42)] are both believed to contribute to a bias away from TH1 differentiation and inflammatory cytokine production in utero. As a result, young infants have heightened vulnerability to numerous infections, particularly those requiring cell-mediated immunity for control, and exhibit delayed development of adaptive immunity to many pathogens (43). It was recently reported that CD4+ T cells with an effector memory phenotype develop during fetal life, even in the absence of any known pathogen exposure, and that these cells perform a variety of inflammatory effector functions (19, 44). However, to date, relatively few studies have performed detailed characterization of effector T cells in the setting of human fetal pathogen exposure. Here, we add to our current understanding of how the fetus is capable of responding to pathogens by demonstrating effector memory differentiation, inflammatory cytokine production, and robust antigen-specific T cell proliferation after in utero exposure to malaria.
Previous studies of pregnancy-associated malaria suggested a role for suppressive Tregs in dampening fetal effector T cell responses to in utero malaria exposure, but the published data are inconsistent. Some studies have reported higher frequencies of Tregs among infants born to mothers with PM and increased production of IFN-γ in response to malaria antigen stimulation after depletion of Tregs (6–11), whereas other studies, including the current study, have found no association between PM and Treg frequency (12,13). Several factors could account for these discordant results, including variability in the definition of PM, as well as differences in the cellular markers and immunologic assays used to identify effector T cells and Tregs (6–13, 45). In addition, the timing, duration, and degree of malaria antigen exposure during pregnancy may influence the fetal T cell response, as may the degree of associated placental inflammation (13, 45). Here, we found that the most profound effector T cell differentiation occurred in infants born to mothers with active PM infection at the time of birth. This may reflect later timing of malaria infection during pregnancy (and hence greater immune maturation of the fetus) or pathogen persistence, as it is not possible to know with confidence when the placenta became infected. Infants born to mothers with evidence of past malaria infection had intermediate frequencies of effector or malaria-responsive T cells. Thus, active PM late during pregnancy or persisting throughout pregnancy may induce the most robust fetal effector T cell responses, whereas malaria infections that were treated or resolved during pregnancy may induce some combination of Tregs and effector T cells (13).
Our study demonstrates a relationship between in utero pathogen exposure, human fetal T cell responses, and protection from infection after birth. Our finding that malaria-specific cord blood CD4+ T cell proliferation correlates with protection from malaria in the first 2 years of life is somewhat surprising, as a protective role for CD4+ T cells in childhood malaria has been difficult to establish (35, 46–50). The relationship between the malaria-specific T cell response and risk of infection is complex, as children residing in highly endemic regions sustain repeated and often prolonged infections, resulting in near-constant exposure to parasites and high-level anti-genemia. Immunoregulatory mechanisms induced by this chronic malaria antigen exposure (including production of the suppressive cytokine IL-10) have been shown to interfere with the generation of fully functional and durable T cell response in children (46). To date, the best proof of principle that sterilizing immunity to malaria in humans is achievable comes from experimental settings in which antigen exposure is limited, either by parasite attenuation or by drug treatment. Such strategies include vaccination with irradiated (or genetically attenuated) sporozoites (51–54), infection under “cover” of blood-stage chemoprevention (55–60), and low-dose blood-stage parasite vaccination (61, 62). These approaches all result in limited exposure to blood-stage parasite antigens and have been associated with the priming of CD4+ T cell responses and induction of sterilizing immunity. It is likely that transplacental exposure of the fetus, as occurs with PM, similarly results in limited exposure to blood-stage antigens and may therefore lead to priming of protective memory CD4+ T cells that can respond vigorously after re-exposure to malaria during infancy.
Our study is limited by the fact that all enrolled women received at least the standard-of-care three-dose IPT for malaria (2, 27), providing some protection from malaria during pregnancy. Although it could be that IPT cleared maternal parasites and altered the fetal T cell response, no differences in fetal T cells were noted between infants born to mothers receiving the standard-of-care versus enhanced IPT. However, it may be that fetal T cell responses induced after highly inflammatory or severe PM in settings of untreated pregnancy-associated malaria would differ from those found in this study. Despite intensive monitoring, it is possible that some maternal infections were missed, resulting in misclassification bias. Some pregnant women and children may have sustained greater malaria exposure than others due to heterogeneous environmental exposure to infected mosquitoes. However, this would likely bias our analysis away from observing a protective response during infancy. Although our work supports an association between in utero malaria exposure, fetal T cell responses, and prospective protection from malaria during childhood, the strength of this finding is limited by a relatively small number of infants (n = 73) included in the protection analyses. Furthermore, we cannot conclude that this relationship is causal, as it is possible that other fetal immune effector mechanisms contribute to this relationship (including B cells and antibodies). It has been shown that malaria infection during pregnancy is associated with the generation of fetal malaria-specific antibodies, indicating that fetal B cells can also be primed in utero (63–65).
Maternal malaria IPT has been shown to decrease infant morbidity (2), and enhanced regimens, recently shown to have excellent efficacy in preventing PM (27, 66), are now being considered for widespread implementation (67). Our data suggest that the immunologic consequences of preventing in utero exposure to malaria require further detailed study. It will be important to determine whether fetal T cell responses to malaria persist and provide durable protection against repeated malaria exposure in childhood. It is possible that malaria antigenemia from breakthrough infections may drive the differentiation of protective CD4+ T cell responses into regulatory IL-10-producing TH1 cells, the predominant CD4+ T cell phenotype observed in highly exposed children (46). Nonetheless, our findings raise the intriguing possibility that vaccination during pregnancy could benefit infants through the induction of fetal effector memory T cell responses. Influenza-specific fetal effector memory CD4+ T cell responses have been identified in cord blood after vaccination during pregnancy (68), providing evidence that transplacental exposure to vaccine antigens, in the absence of active fetal infection, can induce the differentiation of fetal T cells.
In summary, we have identified a population of fetal malaria-responsive T cells in infants born to mothers with active PM that have phenotypic features of effector memory cells and a variety of inflammatory effector functions. These findings advance our understanding of how the fetus can respond to in utero malaria exposure, indicative of a more inflammatory T cell response than previously appreciated. We also show that fetal T cell responses at birth correlate with a reduced risk of malaria in childhood, suggesting in utero priming of a protective T cell response. These results have important implications for understanding the fetal response to pathogens and for strategies that seek to optimize infant immunity.
MATERIALS AND METHODS
Study design
The objective of this study was to evaluate fetal T cell responses in cord blood of infants born to mothers with or without PM. Of the 300 subjects enrolled in the parent clinical trial, 291 were followed until delivery and 271 had cord blood samples collected and banked. Of these available samples, the subset studied here (n = 182) was chosen on the basis of (i) collection and successful banking of at least four vials of CBMCs for an individual, (ii) a recorded concentration of at least 7 × 106 CBMCs per vial, and (iii) a negative result for CMV DNA in a dried blood spot polymerase chain reaction assay (because CMV is another common intrauterine infection that could prime fetal T cells) (69). Variability in the number of cells obtained after thawing resulted in the inability to perform all immunological assays in all individuals (fig. S1). The number of individuals included in each assay is indicated in corresponding figure legends and Materials and methods. Clinical details of the subjects included in this study are outlined in table S2. Primary data for all experiments, where n < 20, are located in table S4.
Ethical approval
Informed consent was obtained from the mother of all study participants. The study protocol was approved by the Uganda National Council of Science and Technology and the institutional review boards of the University of California, San Francisco, Makerere University, and the Centers for Disease Control and Prevention.
Study site and participants
Samples for this study were obtained from a subset of infants enrolled in a clinical trial of prenatal and early childhood malaria IPT in Tororo, Uganda, a rural district in southeastern Uganda with high malaria endemicity. Details of the larger study, including eligibility criteria for enrollment and clinical outcomes, have been previously described in detail (PROMOTE-BC1; NCT02163447) (27). Briefly, 300 HIV-negative pregnant women were enrolled between 12 and 20 weeks of gestation and were randomized to receive standard malaria IPT during pregnancy [three-dose sulfadoxine-pyrimethamine (SP)] versus enhanced IPT (three-dose or monthly DP). Participants randomized to receive standard IPT were administered SP at 20, 28, and 36 weeks of gestation. Similarly, participants randomized to receive three-dose DP were administered drug at 20, 28, and 36 weeks of gestation, whereas those in the monthly DP arm received drug every 4 weeks beginning at 16 or 20 weeks based on gestational age at enrollment. At enrollment, participants received an insecticide-treated bed net, underwent standardized examination, and had blood samples collected. All mothers also received one dose of mebendazole in the second trimester per Ugandan Ministry of Health guidelines. Participants received all of their medical care at the study clinic and had scheduled visits every 4 weeks for routine laboratory testing. Women who presented with fever had a blood smear performed, and if positive, they were treated for malaria (detailed below). Participants were encouraged to deliver at the hospital adjacent to the study clinic.
Screening for maternal parasitemia during pregnancy
Maternal malaria parasitemia was assessed every 4 weeks throughout pregnancy, beginning at enrollment. The presence of malaria parasites in maternal peripheral blood was evaluated using LAMP kits (Eiken Chemical), as previously described (27, 28). LAMP assays were batch analyzed at the end of the trial and were not used to inform treatment (asymptomatic parasitemia is not treated per current Ugandan standard of care). Placental tissue was processed for histopathologic evidence of PM, as previously described (27, 29), and placental blood and cord blood were tested for the presence of malaria parasites by both LAMP and microscopy.
Childhood malaria outcomes
After birth, infants born to enrolled women were randomized to receive DP every 4 or 12 weeks and were followed until 24 months of age. Routine visits were also conducted in children every 4 weeks beginning at birth and included the assessment of peripheral parasitemia by LAMP. Children were encouraged to be brought to the clinic any time they were ill. Those who presented with a documented fever (tympanic temperature > 38.0°C) or history of fever in the previous 24 hours had blood collected for a thick blood smear, and if the smear was positive, the patient was diagnosed with clinical malaria. Episodes of uncomplicated malaria in children <4 months of age or weighing <5 kg, as well as episodes of complicated malaria and treatment failures within 14 days, were treated with a 7-day course of quinine (oral or parenteral).
Infants randomized to receive DP every 4 weeks were excluded from the analysis of prospective protection, as piperaquine has a very long half-life that results in a prophylactic period of ~4 weeks after DP administration (70). All infants randomized to receive DP every 12 weeks during infancy, and for whom sufficient CBMCs were available for performance of the CFSE proliferation assays, were included in the analyses of prospective protection from malaria in childhood (n = 73). This included infants born to mothers with no malaria infection during pregnancy (n = 8), past malaria infection during pregnancy (n = 51), and active PM during pregnancy (n = 13).
Statistical analysis
All statistical analyses were performed using STATA version 14.2 (College Station) and Prism 7.0 (GraphPad). Frequencies of cytokine-producing T cells (alone or in combination) were reported after background subtraction of the frequency of the identically gated population of cells from the same sample stimulated with control. In CFSE dilution assays, the percentage of divided T cells after PfSE stimulation was calculated and reported after subtraction of the percentage of divided T cells after uRBC stimulation. Negative values were graphed as zero. Comparisons of cellular frequencies and proportions between groups were performed using the Kruskal-Wallis test, Wilcoxon rank sum test, or Wilcoxon matched-pairs signed-rank test. The cumulative risk of developing malaria after birth was estimated using the Kaplan-Meier product limit formula. Associations between malaria-responsive CD4+ T cells and the hazard of developing malaria were made using Cox proportional hazards models. The Schoenfeld test was used to test the proportionality of hazards. Negative binomial regression was used to estimate associations between cellular responses at birth and the incidence of malaria (IRRs) and parasite prevalence (PRRs) in the first 2 years of life. Multivariate models were adjusted for maternal malaria exposure (no malaria exposure, past malaria infection, or active PM) (table S1). In all analyses, a two-tailed P value of <0.05 was considered to be statistically significant.
Supplementary Material
www.sciencetranslationalmedicine.org/cgi/content/full/10/463/eaat6176/DC1
Materials and Methods
Fig. S1. Outline of inclusion criteria and experimental prioritization of cord blood samples.
Fig. S2. Fetal effector memory CD4+ T cell frequencies are altered by parasites in the placenta but not by maternal gravidity or IPT arm.
Fig. S3. Fetal effector and memory CD8+ T cell subsets do not differ with PM exposure.
Fig. S4. Cord blood CD4+ T cell responses are fetal in origin.
Fig. S5. Cord blood CD4+ T cell responses are transcriptionally heterogeneous.
Fig. S6. Cytokine production by cord blood CD4+ and CD8+ T cells.
Fig. S7. Characterization of cord blood CD4+ and CD8+ T cell proliferation in response to malaria antigens.
Fig. S8. Gating strategy for CD4+ and CD8+ T cell populations.
Table S1. CD4+ T cell proliferation in response to malaria antigens is associated with protection from childhood malaria with adjustment for maternal malaria exposure.
Table S2. Details of clinical cohort.
Table S3. Details of flow cytometric antibodies.
Table S4. Primary data.
Acknowledgments:
We are grateful to all the study participants and their families for their consent and cooperation in this study. We thank all of the study team members for their tireless dedication and excellent work. We also thank B. Greenhouse, I. Roderiguez-Barraquer, and J. M. McCune for valuable discussions.
Funding: Funding was provided by the NIH/NIAID (5P01HD059454-07 to M.R.K. and G.D., 2R01AI093615-06 and 5K24AI113002-03 to M.E.F., and K23 AI100949 to P.J.).
Footnotes
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data associated with this study are present in the paper or in the Supplementary Materials.
REFERENCES AND NOTES
- 1.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD, Epidemiology and burden of malaria in pregnancy. Lancet Infect. Dis 7, 93–104 (2007). [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization, World Malaria Report 2015 (World Health Organization, 2015), pp. 1–280. [Google Scholar]
- 3.Rogerson SJ, Hviid L, Duffy PE, Leke RFG, Taylor DW, Malaria in pregnancy: Pathogenesis and immunity. Lancet Infect. Dis 7, 105–117 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Malhotra I, Mungai P, Muchiri E, Kwiek JJ, Meshnick SR, King CL, Umbilical cord-blood infections with Plasmodium falciparum malaria are acquired antenatally in Kenya. J. Infect. Dis 194, 176–183 (2006). [DOI] [PubMed] [Google Scholar]
- 5.May K, Grube M, Malhotra I, Long CA, Singh S, Mandaliya K, Siegmund W, Fusch C, Schneider H, King CL, Antibody-dependent transplacental transfer of malaria blood-stage antigen using a human ex vivo placental perfusion model. PLOS ONE 4, e7986 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackroth MS, Malhotra I, Mungai P, Koech D, Muchiri E, King CL, Human cord blood CD4+CD25hi regulatory T cells suppress prenatally acquired T cell responses to Plasmodium falciparum antigens. J. Immunol 186, 2780–2791 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Malhotra I, Dent A, Mungai P, Wamachi A, Ouma JH, Narum DL, Muchiri E, Tisch DJ, King L, Can prenatal malaria exposure produce an immune tolerant phenotype?: A prospective birth cohort study in Kenya. PLOS Med. 6, e1000116 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brustoski K, Möller U, Kramer M, Hartgers FC, Kremsner PG, Krzych U, Luty AJF, Reduced cord blood immune effector-cell responsiveness mediated by CD4+ cells induced in utero as a consequence of placental Plasmodium falciparum infection. J. Infect. Dis 193, 146–154 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Bisseye C, Van Der Sande M, Morgan WD, Holder AA, Pinder M, Ismaili J, Plasmodium falciparum infection of the placenta impacts on the T helper type 1 (Th1)/Th2 balance of neonatal T cells through CD4+CD25+ forkhead box P3+ regulatory T cells and interleukin-10. Clin. Exp. Immunol 158, 287–293 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flanagan KL, Halliday A, Burl S, Landgraf K, Jagne YJ, Noho-Konteh F, Townend J, Miles JC, van der Sande M, Whittle H, Rowland-Jones S, The effect of placental malaria infection on cord blood and maternal immunoregulatory responses at birth. Eur. J. Immunol 40, 1062–1072 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Brustoski K, Möller U, Kramer M, Petelski A, Brenner S, Palmer DR, Bongartz M, Kremsner PG, Luty AJF, Krzych U, IFN-γ and IL-10 mediate parasite-specific immune responses of cord blood cells induced by pregnancy-associated Plasmodium falciparum malaria. J. Immunol 174, 1738–1745 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Soulard V, Amadoudji Zin M, Fitting C, Ibitokou S, Oesterholt M, Luty AJF, Perrin R-X, Massougbodji A, Deloron P, Bandeira A, Fievet N, Placental malaria-associated suppression of parasite-specific immune response in neonates has no major impact on systemic CD4 T cell homeostasis. Infect. Immun 79, 2801–2809 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prahl M, Jagannathan P, McIntyre TI, Auma A, Farrington L, Wamala S, Nalubega M, Musinguzi K, Naluwu K, Sikyoma E, Budker R, Vance H, Odorizzi P, Nayebare P, Ategeka J, Kakuru A, Havlir DV, Kamya MR, Dorsey G, Feeney ME, Timing of in utero malaria exposure influences fetal CD4 T cell regulatory versus effector differentiation. Malar. J 15, 497 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mold JE, Michaëlsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee T-H, Nixon DF, Mccune JM, Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 322, 1562–1565 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adkins B, Leclerc C, Marshall-Clarke S, Neonatal adaptive immunity comes of age. Nat. Rev. Immunol 4, 553–564 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Rose S, Lichtenheld M, Foote MR, Adkins B, Murine neonatal CD4+ cells are poised for rapid Th2 effector-like function. J. Immunol 178, 2667–2678 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White GP, Watt PM, Holt BJ, Holt PG, Differential patterns of methylation of the IFN-γ promoter at CpG and non-CpG sites underlie differences in IFN-γ gene expression between human neonatal and adult CD45RO-T cells. J. Immunol 168, 2820–2827 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Kaminuma O, Kitamura F, Miyatake S, Yamaoka K, Miyoshi H, Inokuma S, Tatsumi H, Nemoto N Kitamura, Akio Mori, Takachika Hiroi, T-box 21 transcription factor is responsible for distorted TH2 differentiation in human peripheral CD4+ T cells. J. Allergy Clin. Immunol 123, 813–823.e3 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Mozeleski B, Lemoine S, Dériaud E, Lim A, Zhivaki D, Azria E, Le Ray C, Roguet G, Launay O, Vanet A, Leclerc C, Lo-Man R, CD4 T cells with effector memory phenotype and function develop in the sterile environment of the fetus. Sci. Transl. Med 6, 238ra72 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Hong M, Sandalova E, Low D, Gehring AJ, Fieni S, Amadei B, Urbani S, Chong Y-S, Guccione E, Bertoletti A, Trained immunity in newborn infants of HBV-infected mothers. Nat. Commun 6, 6588 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babik JM, Cohan D, Monto A, Hartigan-O’Connor DJ, Mccune JM, The human fetal immune response to hepatitis C virus exposure in utero. J. Infect. Dis 203, 196–206 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luzuriaga K, Holmes D, Hereema A, Wong J, Panicali DL, Sullivan JL, HIV-1-specific cytotoxic T lymphocyte responses in the first year of life. J. Immunol 154, 433–443 (1995). [PubMed] [Google Scholar]
- 23.Sanchez-Merino V, Nie S, Luzuriaga K, HIV-1-specific CD8+ T cell responses and viral evolution in women and infants. J. Immunol 175, 6976–6986 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Legrand FA, Nixon DF, Loo CP, Ono E, Chapman JM, Miyamoto M, Diaz RS, Santos AMN, Succi RCM, Abadi J, Rosenberg MG, de Moraes-Pinto MI, Kallas EG, Strong HIV-1-specific T cell responses in HIV-1-exposed uninfected infants and neonates revealed after regulatory T cell removal. PLOS ONE 1, e102 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huygens A, Lecomte S, Tackoen M, Olislagers V, Delmarcelle Y, Burny W, Van Rysselberge M, Liesnard C, Larsen M, Appay V, Donner C, Marchant A, Functional exhaustion limits CD4+ and CD8+ T-cell responses to congenital cytomegalovirus infection. J. Infect. Dis 212, 484–494 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Marchant A, Appay V, van der Sande M, Dulphy N, Liesnard C, Kidd M, Kaye S, Ojuola O, Gillespie GMA, Vargas Cuero AL, Cerundolo V, Callan M, McAdam KPWJ, Rowland-Jones SL, Donner C, McMichael AJ, Whittle H, Mature CD8+ T lymphocyte response to viral infection during fetal life. J. Clin. Invest 111, 1747–1755 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kakuru A, Jagannathan P, Muhindo MK, Natureeba P, Awori P, Nakalembe M, Opira, Olwoch P, Ategeka J, Nayebare P, Clark TD, Feeney ME, Charlebois ED, Rizzuto G, Muehlenbachs A, Havlir DV, Kamya MR, Dorsey G, Dihydroartemisinin-piperaquine for the prevention of malaria in pregnancy. N. Engl. J. Med 374, 928–939 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hopkins H, González IJ, Polley SD, Angutoko P, Ategeka J, Asiimwe C, Agaba B, Kyabayinze DJ, Sutherland CJ, Perkins MD, Bell D, Highly sensitive detection of malaria parasitemia in a malaria-endemic setting: Performance of a new loop-mediated isothermal amplification kit in a remote clinic in Uganda. J. Infect. Dis 208, 645–652 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogerson SJ, Mwapasa V, Meshnick SR, Malaria in pregnancy: Linking immunity and pathogenesis to prevention. Am. J. Trop. Med. Hyg 77, 14–22 (2007). [PubMed] [Google Scholar]
- 30.Harrington WE, Kanaan SB, Muehlenbachs A, Morrison R, Stevenson P, Fried M, Duffy PE, Nelson JL, Maternal microchimerism predicts increased infection but decreased disease due to Plasmodium falciparum during early childhood. J. Infect. Dis 215, 1445–1451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanaan SB, Gammill HS, Harrington WE, De Rosa SC, Stevenson PA, Forsyth AM, Allen J, Cousin E, van Besien K, Delaney CS, Nelson JL, Maternal microchimerism is prevalent in cord blood in memory T cells and other cell subsets, and persists post-transplant. OncoImmunology 6, e1311436 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becattini S, Latorre D, Mele F, Foglierini M, De Gregorio C, Cassotta A, Fernandez B, Kelderman S, Schumacher TN, Corti D, Lanzavecchia A, Sallusto F, Functional heterogeneity of human memory CD4+ T cell clones primed by pathogens or vaccines. Science 347, 400–406 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Engelmann I, Santamaria A, Kremsner PG, Luty AJF, Activation status of cord blood γδ T cells reflects in utero exposure to Plasmodium falciparum antigen. J. Infect. Dis 191, 1612–1622 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Cairo C, Longinaker N, Cappelli G, Leke RGF, Ondo MM, Djokam R, Fogako J, Leke RJ, Sagnia B, Sosso S, Colizzi V, Pauza CD, Cord blood Vγ2Vδ2 T cells provide a molecular marker for the influence of pregnancy-associated malaria on neonatal immunity. J. Infect. Dis 209, 1653–1662 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jagannathan P, Nankya F, Stoyanov C, Eccles-James I, Sikyomu E, Naluwu K, Wamala S, Nalubega M, Briggs J, Bowen K, Bigira V, Kapisi J, Kamya MR, Dorsey G, Feeney ME, IFNγ responses to pre-erythrocytic and blood-stage malaria antigens exhibit differential associations with past exposure and subsequent protection. J. Infect. Dis 211, 1987–1996 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webster RB, Rodriguez Y, Klimecki WT, Vercelli D, The human IL-13 locus in neonatal CD4+ T cells is refractory to the acquisition of a repressive chromatin architecture. J. Biol. Chem 282, 700–709 (2006). [DOI] [PubMed] [Google Scholar]
- 37.De Wit D, Olislagers V, Goriely S, Vermeulen F, Wagner H, Goldman M, Willems F, Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood 103, 1030–1032 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Renneson J, Dutta B, Goriely S, Danis B, Lecomte S, Laes J-F, Tabi Z, Goldman M, Marchant A, IL-12 and type I IFN response of neonatal myeloid DC to human CMV infection. Eur. J. Immunol 39, 2789–2799 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Goriely S, Vincart B, Stordeur P, Vekemans J, Willems F, Goldman M, De Wit D, Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol 166, 2141–2146 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Goriely S, Van Lint C, Dadkhah R, Libin M, De Wit D, Demonté D, Willems F, Goldman M, A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal dendritic cells. J. Exp. Med 199, 1011–1016 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aksoy E, Albarani V, Nguyen M, Laes J-F, Ruelle J-L, De Wit D, Willems F, Goldman M, Goriely S, Interferon regulatory factor 3-dependent responses to lipopolysaccharide are selectively blunted in cord blood cells. Blood 109, 2887–2893 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Krow-Lucal ER, Kim CC, Burt TD, McCune JM, Distinct functional programming of human fetal and adult monocytes. Blood 123, 1897–1904 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE; Child Health Epidemiology Reference Group of WHO and UNICEF, Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 379, 2151–2161 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Frascoli M, Coniglio L, Witt R, Jeanty C, Fleck-Derderian S, Myers DE, Lee T-H, Keating S, Busch MP, Norris PJ, Tang Q, Cruz G, Barcellos LF, Gomez-Lopez N, Romero R, MacKenzie TC, Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-γ and TNF-α. Sci. Transl. Med 10, eaan2263 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Odorizzi PM, Feeney ME, Impact of in utero exposure to malaria on fetal T cell immunity. Trends Mol. Med 22, 877–888 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jagannathan P, Eccles-James I, Bowen K, Nankya F, Auma A, Wamala S, Ebusu C, Muhindo MK, Arinaitwe E, Briggs J, Greenhouse B, Tappero JW, Kamya MR, Dorsey ME Feeney, IFNγ/IL-10 co-producing cells dominate the CD4 response to malaria in highly exposed children. PLOS Pathog. 10, e1003864 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyle MJ, Jagannathan P, Farrington LA, Eccles-James I, Wamala S, McIntyre TI, Vance M, Bowen K, Nankya F, Auma A, Nalubega M, Sikyomu E, Naluwu K, Rek J, Katureebe, Bigira V, Kapisi J, Tappero J, Muhindo MK, Greenhouse B, Arinaitwe E, Dorsey G, Kamya MR, Feeney ME, Decline of FoxP3+ regulatory CD4 T cells in peripheral blood of children heavily exposed to malaria. PLOS Pathog. 11, e1005041 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyle MJ, Jagannathan P, Bowen K, McIntyre TI, Vance HM, Farrington LA, Greenhouse, Nankya F, Rek J, Katureebe A, Arinaitwe E, Dorsey G, Kamya MR, Feeney ME, Effector phenotype of Plasmodium falciparum-specific CD4+ T cells is influenced by both age and transmission intensity in naturally exposed populations. J. Infect. Dis 212, 416–425 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Obeng-Adjei N, Portugal S, Tran TM, Yazew TB, Skinner J, Li S, Jain A, Felgner PL, Doumbo OK, Kayentao K, Ongoiba A, Traore B, Crompton PD, Circulating Th1-cell-type Tfh cells that exhibit impaired B cell help are preferentially activated during acute malaria in children. Cell Rep. 13, 425–439 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT, Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat. Immunol 13, 188–195 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Epstein JE, Tewari K, Lyke KE, Sim BKL, Billingsley PF, Laurens MB, Gunasekera A, Chakravarty S, James ER, Sedegah M, Richman A, Velmurugan S, Reyes S, Li M, Tucker K, Ahumada A, Ruben AJ, Li T, Stafford R, Eappen AG, Tamminga C, Bennett JW, Ockenhouse CF, Murphy JR, Komisar J, Thomas N, Loyevsky M, Birkett A, Plowe CV, Loucq C, Edelman R, Richie TL, Seder RA, Hoffman SL, Live attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science 334, 475–480 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Ishizuka AS, Lyke KE, DeZure A, Berry AA, Richie TL, Mendoza FH, Enama ME, Gordon J, Chang L-J, Sarwar UN, Zephir KL, Holman LA, James ER, Billingsley PF, Gunasekera A, Chakravarty S, Manoj A, Li M, Ruben AJ, Li T, Eappen G, Stafford RE, Natasha KC, Murshedkar T, DeCederfelt H, Plummer SH, Hendel S, Novik L, Costner PJM, Saunders JG, Laurens MB, Plowe CV, Flynn B, Whalen WR, Todd JP, Noor J, Rao S, Sierra-Davidson K, Lynn GM, Epstein JE, Kemp MA, Fahle GA, Mikolajczak SA, Fishbaugher M, Sack BK, Kappe SHI, Davidson SA, Garver LS, Björkström NK, Nason MC, Graham BS, Roederer M, Sim KL, Hoffman SL, Ledgerwood JE, Seder RA, Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat. Med 22, 614–623 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mordmüller B, Surat G, Lagler H, Chakravarty S, Ishizuka AS, Lalremruata A, Gmeiner M, Campo JJ, Esen M, Ruben AJ, Held J, Calle CL, Mengue JB, Gebru T, Ibáñez J, Sulyok M, James ER, Billingsley PF, Natasha KC, Manoj A, Murshedkar T, Gunasekera, Eappen AG, Li T, Stafford RE, Li M, Felgner PL, Seder RA, Richie TL, Sim KL, Hoffman SL, Kremsner PG, Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 542, 445–449 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doll KL, Harty JT, Correlates of protective immunity following whole sporozoite vaccination against malaria. Immunol. Res 59, 166–176 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roestenberg M, Teirlinck AC, McCall MBB, Teelen K, Makamdop KN, Wiersma J, Arens T, Beckers P, van Gemert G, van de Vegte-Bolmer M, van der Ven AJAM, Luty JF, Hermsen CC, Sauerwein RW, Long-term protection against malaria after experimental sporozoite inoculation: An open-label follow-up study. Lancet 377, 1770–1776 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJF, van Gemert GJ, van de Vegte-Bolmer M, van Schaijk B, Teelen K, Arens T, Spaarman L, de Mast Q, Roeffen W, Snounou G, Rénia L, van der Ven AJ, Hermsen CC, Sauerwein RW, Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med 361, 468–477 (2009). [DOI] [PubMed] [Google Scholar]
- 57.Belnoue E, Costa FTM, Frankenberg T, Vigário AM, Voza T, Leroy N, Rodrigues MM, Landau I, Snounou G, Rénia L, Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J. Immunol 172, 2487–2495 (2004). [DOI] [PubMed] [Google Scholar]
- 58.Jagannathan P, Bowen K, Nankya F, McIntyre TI, Auma A, Wamala S, Sikyomu E, Naluwu K, Nalubega M, Boyle MJ, Farrington LA, Bigira V, Kapisi J, Aweeka F, Greenhouse, Kamya M, Dorsey G, Feeney ME, Effective antimalarial chemoprevention in childhood enhances the quality of CD4+ T cells and limits their production of immunoregulatory interleukin 10. J. Infect. Dis 214, 329–338 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Putrianti ED, Silvie O, Kordes M, Borrmann S, Matuschewski K, Vaccine-like immunity against malaria by repeated causal-prophylactic treatment of liver-stage Plasmodium parasites. J. Infect. Dis 199, 899–903 (2009). [DOI] [PubMed] [Google Scholar]
- 60.Friesen J, Silvie O, Putrianti ED, Hafalla JCR, Matuschewski K, Borrmann S, Natural immunization against malaria: Causal prophylaxis with antibiotics. Sci. Transl. Med 40, 40ra49 (2010). [DOI] [PubMed] [Google Scholar]
- 61.Pinzon-Charry A, McPhun V, Kienzle V, Hirunpetcharat C, Engwerda C, McCarthy J, Good MF, Low doses of killed parasite in CpG elicit vigorous CD4+ T cell responses against blood-stage malaria in mice. J. Clin. Invest 120, 2967–2978 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pombo DJ, Lawrence G, Hirunpetcharat C, Rzepczyk C, Bryden M, Cloonan N, Anderson K, Mahakunkijcharoen Y, Martin LB, Wilson D, Elliott S, Elliott S, Eisen DP, Weinberg JB, Saul A, Good MF, Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet 360, 610–617 (2002). [DOI] [PubMed] [Google Scholar]
- 63.Tassi Yunga S, Kayatani AK, Fogako J, Leke RJI, Leke RGF, Taylor DW, Timing of the human prenatal antibody response to Plasmodium falciparum antigens. PLOS ONE 12, e0184571 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dent A, Malhotra I, Mungai P, Muchiri E, Crabb BS, Kazura JW, King CL, Prenatal malaria immune experience affects acquisition of Plasmodium falciparum merozoite surface protein-1 invasion inhibitory antibodies during infancy. J. Immunol 177, 7139–7145 (2006). [DOI] [PubMed] [Google Scholar]
- 65.King CL, Malhotra I, Wamachi A, Kioko J, Mungai P, Wahab SA, Koech D, Zimmerman P, Ouma J, Kazura JW, Acquired immune responses to Plasmodium falciparum merozoite surface protein-1 in the human fetus. J. Immunol 168, 356–364 (2002). [DOI] [PubMed] [Google Scholar]
- 66.Desai M, Gutman J, L’lanziva A, Otieno K, Juma E, Kariuki S, Ouma P, Were V, Laserson K, Katana A, Williamson J, ter Kuile FO, Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin-piperaquine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for the control of malaria during pregnancy in western Kenya: An open-label, three-group, randomised controlled superiority trial. Lancet 386, 2507–2519 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gutman J, Kovacs S, Dorsey G, Stergachis A, ter Kuile FO, Safety, tolerability, and efficacy of repeated doses of dihydroartemisinin-piperaquine for prevention and treatment of malaria: A systematic review and meta-analysis. Lancet Infect. Dis 17, 184–193 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rastogi D, Wang C, Mao X, Lendor C, Rothman PB, Miller RL, Antigen-specific immune responses to influenza vaccine in utero. J. Clin. Invest 117, 1637–1646 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boppana SB, Ross SA, Novak Z, Shimamura M, Tolan RW, Palmer AL, Ahmed A, Michaels MG, Sánchez PJ, Bernstein DI, Britt WJ, Fowler KB; National Institute on Deafness and Other Communication Disorders CMV and Hearing Multicenter Screening (CHIMES) Study, Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA 303, 1375–1382 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nankabirwa J, Cundill B, Clarke S, Kabatereine N, Rosenthal PJ, Dorsey G, Brooker S, Staedke SG, Efficacy, safety, and tolerability of three regimens for prevention of malaria: A randomized, placebo-controlled trial in Ugandan schoolchildren. PLOS ONE 5, e13438 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
www.sciencetranslationalmedicine.org/cgi/content/full/10/463/eaat6176/DC1
Materials and Methods
Fig. S1. Outline of inclusion criteria and experimental prioritization of cord blood samples.
Fig. S2. Fetal effector memory CD4+ T cell frequencies are altered by parasites in the placenta but not by maternal gravidity or IPT arm.
Fig. S3. Fetal effector and memory CD8+ T cell subsets do not differ with PM exposure.
Fig. S4. Cord blood CD4+ T cell responses are fetal in origin.
Fig. S5. Cord blood CD4+ T cell responses are transcriptionally heterogeneous.
Fig. S6. Cytokine production by cord blood CD4+ and CD8+ T cells.
Fig. S7. Characterization of cord blood CD4+ and CD8+ T cell proliferation in response to malaria antigens.
Fig. S8. Gating strategy for CD4+ and CD8+ T cell populations.
Table S1. CD4+ T cell proliferation in response to malaria antigens is associated with protection from childhood malaria with adjustment for maternal malaria exposure.
Table S2. Details of clinical cohort.
Table S3. Details of flow cytometric antibodies.
Table S4. Primary data.