Abstract
Research suggests that the prevalence and incidence of cognitive impairment among older adults is decreasing. This analysis used data from 9 waves (1993–2016) of the Hispanic Established Populations for the Epidemiologic Study of the Elderly to assess cognitive status and cognitive decline for 2 cohorts of Mexican-Americans aged ≥75 years in 1993–1994 versus 2004–2005. Logistic regression, joint longitudinal survival models, and illness-death models for interval-censored data were used to examine cohort differences in the odds of prevalent cognitive impairment, trajectories of cognitive decline, and the risk of 10-year incident cognitive impairment, respectively. Results indicated that compared with the 1993–1994 cohort, the 2004–2005 cohort had higher odds for prevalent cognitive impairment (odds ratio = 2.51, 95% confidence interval (CI): 1.92, 3.29), particularly among participants with <4 years of education (odds ratio = 2.99, 95% CI: 2.14, 4.18). Conversely, the 2004–2005 cohort exhibited significantly slower rates of cognitive decline ( = 0.50, 95% CI: 0.39, 0.62) and had a significantly lower risk of incident cognitive impairment (hazard ratio = 0.75, 95% CI: 0.62, 0.91) compared with the 1993–1994 cohort. This analysis provides mixed results for cohort trends in the cognitive health of older Mexican-Americans. Continued research is needed to identify risk factors that contribute to these population-level trends.
Keywords: cognitive impairment, incidence, Mexican-Americans, prevalence
Cognitive impairment and Alzheimer disease and related dementias (ADRD) are major public health concerns. Population aging will cause an increase in the number of older adults living with ADRD (1). However, several studies have observed decreasing trends in ADRD prevalence and incidence (2–12). These findings have been attributed to improved treatment of chronic diseases and higher educational attainment among older adults (13, 14). While not all studies have reported favorable trends in ADRD (15–17), cognitive impairment (18), or cognitive function (19, 20), these results suggest that promoting education, improving management of chronic diseases, and reducing vascular risk factors might be effective strategies for preventing ADRD (10).
Racial and ethnic disparities in cognitive impairment and ADRD are well documented (21–25), although only a few trend studies have focused on minority populations (26–29). Investigators using data from the Health and Retirement Study reported that the prevalence of cognitive impairment from 1993 to 2004 among adults aged ≥70 years decreased 3.9% per year for non-Hispanic white, 5.2% for black, and 4.7% for Hispanic persons (29). Findings from the Washington Heights–Inwood Columbia Aging Project revealed the risk of dementia among adults aged ≥65 years in 1999 compared with 1992 was 40% lower for non-Hispanic white, 48% lower for black, and 36% lower for Hispanic persons (28). Similarly, the age-specific incidence rate for dementia in 2 cohorts of African Americans aged ≥70 years from Indianapolis decreased from 3.6% in 1992 to 1.4% in 2001 (26). Conversely, an increase in the prevalence of cognitive impairment among Mexican-Americans aged ≥75 years has been reported (27).
Hispanic Americans are the largest minority population in the United States (30). Hispanics have longer life expectancy than black and non-Hispanic white Americans (31) despite substantial disadvantages in socioeconomic and health characteristics (32, 33). This make older Hispanics a high-risk population for cognitive impairment and ADRD. This analysis uses data from the Hispanic Established Populations for the Epidemiologic Study of the Elderly (H-EPESE) to examine differences in the prevalence and incidence of cognitive impairment, and trajectories of cognitive decline between Mexican-Americans aged ≥75 years in 2004–2005 and 1993–1994. We hypothesized that: 1) Older Mexican-Americans in 2004–2005 would have higher odds of being cognitively impaired; 2) the 2004–2005 cohort would exhibit slower cognitive decline; and 3) the risk of incident cognitive impairment would be lower for the 2004–2005 cohort.
METHODS
Sample
The H-EPESE is a longitudinal study of Mexican-Americans aged ≥65 years, living in the Southwestern United States (Texas, California, Arizona, Colorado, and New Mexico) (34). The first observation wave was completed in 1993–1994, and 9 observation waves have been completed as of 2016. Details on the sampling procedures have been previously described (35, 36). A multistage area-probability cluster sample was used to select census tracts in counties in which the Mexican-American population comprised at least 6.6% of the county population. Census blocks were then randomly selected to identify Mexican-Americans aged ≥65 years from a minimum of 400 households in each census tract. The baseline observation wave in 1993–1994 had a response rate of 83% and was representative of 500,000 Mexican-Americans aged ≥65 years living in the Southwestern United States (35). A new independent sample of 902 participants aged ≥75 years was enrolled at wave 5 (2004–2005). This new cohort was added so that the H-EPESE reflected the increasing educational attainment and income among older Mexican-Americans (37). New participants were selected using sampling procedures consistent with those used in 1993–1994. Weights were calculated so that the new sample was representative of Mexican-Americans aged ≥75 years living in the Southwestern United States (37).
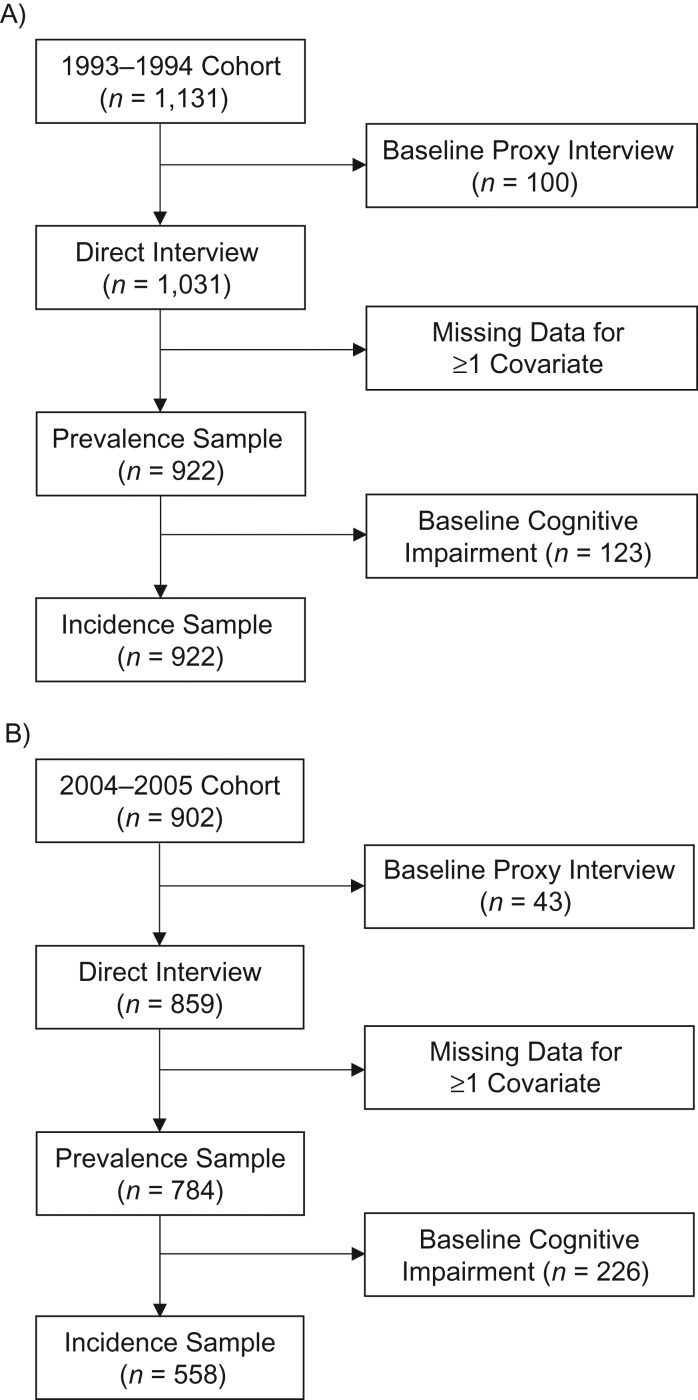
The selection of the analytical sample for each cohort is presented in Figure 1. Participants who required a proxy to complete the baseline interview or were missing data for 1 or more covariates at the baseline interview were excluded. Participants who required a proxy to complete the baseline interview were excluded because the H-EPESE does not include a proxy measure for cognition, and not all of the covariates included in this analysis are assessed by proxy interview. The analytical sample for analyses on prevalent cognitive impairment and trajectories of cognitive decline included 1,706 participants, 922 from the 1993–1994 cohort and 784 from the 2004–2005 cohort. The analytical sample for incident cognitive impairment included 1,357 participants, 799 from the 1993–1994 cohort and 558 from the 2004–2005 cohort.
Figure 1.
Selection of the final analytical sample from the Hispanic Established Populations for the Epidemiologic Study of the Elderly, United States. A) 1993–1994 cohort; B) 2004–2005 cohort.
Waves 1–5 were used for the 1993–1994 cohort and waves 5–9 were used for the 2004–2005 cohort. The 2004–2005 cohort included only participants who entered into the H-EPESE at wave 5. A total of 882 participants who were <75 years of age at wave 1 were also interviewed at wave 5. We excluded these 882 participants from the 2004–2005 cohort so that the maximum number of times in which participants could be observed was identical for the 1993–1994 cohort and 2004–2005 cohort. The 882 surviving participants were 1.4 years younger than participants included in the 2004–2005 cohort (P < 0.01), but there were no statistically significant differences in sex, education, or self-reported health conditions.
Measures
Outcomes of interest
Cognitive functioning of participants who did not require a proxy to complete the interview was assessed using the Mini-Mental State Examination (MMSE) (38). Prior research has noted floor and ceiling effects that limit the MMSE in accurately measuring the cognitive functioning of older adults with very high or very low cognition (39, 40). Philipps et al. (41) have developed a methodology using latent process models (42, 43) to obtain transformed MMSE scores that minimize potential biases from floor/ceiling effects and account for the nonlinear relationship between a respondent’s initial MMSE score and the rate of change over time (41). The range of possible scores on the MMSE is 0–30, and the transformed scores are rescaled to a range of 0–100 points (41). The normalized transformation of the raw MMSE scores can be obtained using the R (R Foundation for Statistical Computing, Vienna, Austria) package NormPsy (44). Results for cohort differences in the trajectories of cognitive decline according to the raw and normalized MMSE scores are presented.
Cognitive impairment for participants who did not require a proxy to complete the interview was defined as scoring ≤18 points on the MMSE, to account for the low education and advanced age of the sample population. Incident cases of cognitive impairment were ascertained by identifying the first observation wave in which a participant scored ≤18 points on the MMSE.
For the 1993–1994 cohort, the percentages of participants who required a proxy interview for waves 1–5 were 8.8%, 8.7%, 12.7%, 11.2%, and 14.1%, respectively. For the 2004–2005 cohort, the percentages of proxy interviews for waves 5–9 were 4.8%, 9.8%, 1.9%, 5.8%, and 11.7%, respectively. The H-EPESE survey specifies whether a proxy interview was required because of impaired cognition. Proxy respondents are also asked if the target participant has been diagnosed by a physician with Alzheimer disease.
Covariates
Selected covariates included age, sex, education, being born in the United States or Mexico (i.e., nativity), diabetes, heart disease, hypertension, stroke, and depression. All covariates were selected from the respective baseline observation waves. Diabetes, heart disease, hypertension, and stroke were based on self-report. Participants who reported never having been diagnosed with hypertension but who had a systolic blood pressure of ≥140 mm Hg or diastolic blood pressure of ≥90 mm Hg were also classified as having hypertension. Depression was defined as scoring ≥16 points on the Center for Epidemiologic Studies–Depression Scale (45).
Analysis
Independent sample t tests and χ2 tests were used to assess differences in baseline characteristics between the 1993–1994 cohort and 2004–2005 cohort. Logistic regression models were used to assess the odds of prevalent cognitive impairment among older Mexican-Americans in 2004–2005 compared with 1993–1994. Logistic regression models were also used to estimate the predicted probability of prevalent cognitive impairment in the 1993–1994 cohort and 2004–2005 cohort. The risk difference based on each logistic regression model was calculated by subtracting the predicted probability of cognitive impairment for the 2004–2005 cohort from the predicted probability of cognitive impairment for the 1993–1994 cohort.
The trajectories of cognitive decline were assessed using joint longitudinal survival models (46). This approach was used because rates of cognitive decline increase prior to death (47, 48). A joint longitudinal survival model uses submodels to simultaneously estimate the trajectories of cognitive decline and risk of mortality. The cognitive trajectories were modeled using linear mixed-effects regression (49). This approach produces valid estimates when data is unbalanced because of differences in the number or timing of the observations of the participants (49, 50). Random effects for time (years since baseline) and intercept were included to allow for the trajectory and baseline estimates for cognitive functioning to vary for each participant. A term for the interaction of cohort × time was included to determine whether cognitive trajectories differed between the 1993–1994 cohort and 2004–2005 cohort. The cohort × time term significantly improved the fit of the linear mixed-effects regression models according to the Akaike information criterion. These models also included a dummy variable to indicate the first observation wave (e.g., 0, 1, 1, 1, 1) to account for potential practice effects on the MMSE (51). The survival model was estimated using Cox proportional hazards regression. The joint models were estimated using the R (R Foundation for Statistical Computing) package JM (52).
The risk of incident cognitive impairment was examined using an illness-death model for interval-censored data (53, 54). This approach allows for participants who remained cognitively intact to develop cognitive impairment between their last observation and death. The model estimates 3 transitions: 1) cognitively intact to deceased; 2) cognitively intact to cognitively impaired; and 3) cognitively impaired to deceased. These estimates are based on the age at which a participant is last observed to be cognitively intact, the age at which a participant is first observed to be cognitively impaired, and age at death. The illness-death models were estimated using the R (R Foundation for Statistical Computing) package SmoothHazard (55).
Covariates were added into the analyses using a series of models. Model 1 controlled for age (centered at the sample mean), sex (referent: male), and nativity (referent: foreign-born). A model for the risk of incident cognitive impairment that controlled for baseline MMSE score is also presented. Model 2 added years of education. Years of education was dichotomized as ≥4 and <4 years for regression models that included a term for interaction of education × cohort. Model 3 added diabetes, heart disease, hypertension, stroke, and depression. A fourth model for the trajectories of cognitive decline included terms for interaction between time and each covariate. Subsequent analyses were conducted to test for interactions between cohort and each covariate to determine whether cohort differences in the odds of prevalent cognitive impairment, trajectories of cognitive decline, and risk of incident cognitive impairment varied according to specific sociodemographic and health characteristics. The H-EPESE sampling weights were used to account for the survey design. All analyses were completed using R (R Foundation for Statistical Computing), version 3.1.0 (56).
RESULTS
The descriptive characteristics of the final sample are presented in Table 1. Participants in the 2004–2005 cohort were older, were more likely to be born in the United States, completed more years of education, and were more likely to have diabetes or hypertension, to be cognitively impaired, and to have lower mean scores on the raw and transformed MMSE. The 1993–1994 cohort was more likely to have experienced a stroke and depression. Among all US-born participants, the 2004–2005 cohort completed an average of 6.3 years of education compared with 5.1 years for the 1993–1994 cohort (P < 0.01). The mean years of education among all foreign-born participants was 3.5 for the 2004–2005 cohort and 3.6 for the 1993–1994 cohort (P = 0.59).
Table 1.
Baseline Characteristics of 2 Cohorts, Hispanic Established Populations for the Epidemiologic Study of the Elderlya, United States, 1993–1994 and 2004–2005
| Characteristic | 1993–1994 Cohort (n = 922) | 2004–2005 Cohort (n = 784) | P Value | ||
|---|---|---|---|---|---|
| No. of Persons | % | No. of Persons | % | ||
| Age, yearsb | 80.5 (0.22) | 81.2 (0.22) | 0.02 | ||
| Female sex | 544 | 58.6 | 461 | 59.7 | 0.61 |
| US born | 455 | 44.8 | 436 | 55.9 | <0.01 |
| Years of educationb | 4.3 (0.16) | 5.0 (0.22) | <0.01 | ||
| Diabetes | 281 | 22.6 | 277 | 36.2 | <0.01 |
| Heart disease | 124 | 14.5 | 88 | 13.4 | 0.54 |
| Hypertension | 575 | 65.5 | 549 | 72.1 | <0.01 |
| Stroke | 78 | 10.6 | 62 | 7.4 | 0.02 |
| Depression | 228 | 26.2 | 143 | 21.5 | 0.02 |
| Cognitively impaired | 123 | 15.3 | 226 | 27.9 | <0.01 |
| Raw MMSE scoreb | 23.1 (0.35) | 21.1 (0.26) | <0.01 | ||
| Transformed MMSE scoreb | 55.5 (1.12) | 47.6 (0.93) | <0.01 | ||
Abbreviation: MMSE, Mini-Mental State Examination.
a Percentages and standard errors were calculated using the sample weights from the Hispanic Established Populations for the Epidemiologic Study of the Elderly.
b Values are expressed as mean (standard error).
Prevalent cognitive impairment
The 2004–2005 cohort had 2.11 (95% confidence interval (CI): 1.65, 2.71) times higher odds of being cognitively impaired at baseline compared with the 1993–1994 cohort independent of age, sex, and nativity (Table 2). The odds of cognitive impairment increased to 2.37 (95% CI: 1.82, 3.11) after controlling for education and to 2.51 (95% CI: 1.92, 3.29) after controlling for health characteristics. The risk differences between the 2004–2005 cohort and 1993–1994 cohort for models 1–3 were 11.95 (95% CI: 8.12, 15.78), 13.29 (95% CI: 9.51, 17.07), and 13.74 (95% CI: 10.00, 17.47), respectively (Table 2).
Table 2.
Odds Ratios for Cognitive Impairment Among Mexican-Americans Aged ≥75 Years, Hispanic Established Populations for the Epidemiologic Study of the Elderlya, United States, 2004–2005 Cohort Compared With 1993–1994 Cohort
| Variable | Model 1 | Model 2 | Model 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | Risk Difference | 95% CI | OR | 95% CI | Risk Difference | 95% CI | OR | 95% CI | Risk Difference | 95% CI | |
| 2004–2005 cohort overall | 2.11 | 1.65, 2.71 | 11.95 | 8.12, 15.78 | 2.37 | 1.82, 3.11 | 13.29 | 9.51, 17.07 | 2.51 | 1.92, 3.29 | 13.74 | 10.00, 17.47 |
| Age | 1.10 | 1.07, 1.13 | 1.09 | 1.07, 1.12 | 1.10 | 1.07, 1.13 | ||||||
| Female sex | 1.17 | 0.91, 1.51 | 1.20 | 0.92, 1.57 | 1.11 | 0.85, 1.46 | ||||||
| US born | 0.83 | 0.65, 1.07 | 1.02 | 0.78, 1.34 | 0.99 | 0.75, 1.29 | ||||||
| Education | 0.87 | 0.84, 0.90 | 0.87 | 0.84, 0.91 | ||||||||
| Diabetes | 1.28 | 0.96, 1.69 | ||||||||||
| Heart disease | 0.82 | 0.55, 1.20 | ||||||||||
| Hypertension | 0.70 | 0.53, 0.93 | ||||||||||
| Stroke | 2.92 | 1.93, 4.39 | ||||||||||
| Depression | 1.55 | 1.15, 2.08 | ||||||||||
Abbreviations: CI, confidence interval; OR, odds ratio.
a Results are from multivariable logistic regression models. Model 1 controlled for age, sex, and nativity. Model 2 controlled for covariates in model 1 plus education. Model 3 controlled for covariates in model 2 plus diabetes, heart disease, hypertension, stroke, and depression.
The results from the interaction analyses revealed that the increased odds of prevalent cognitive impairment for the 2004–2005 cohort varied according to educational attainment (P for interaction = 0.03). The 2004–2005 cohort had 1.85 (95% CI: 1.20, 2.88) times higher odds of cognitive impairment among participants with ≥4 years of education compared with 2.99 (95% CI: 2.14, 4.18) higher odds among participants with low education.
Trajectories of cognitive decline
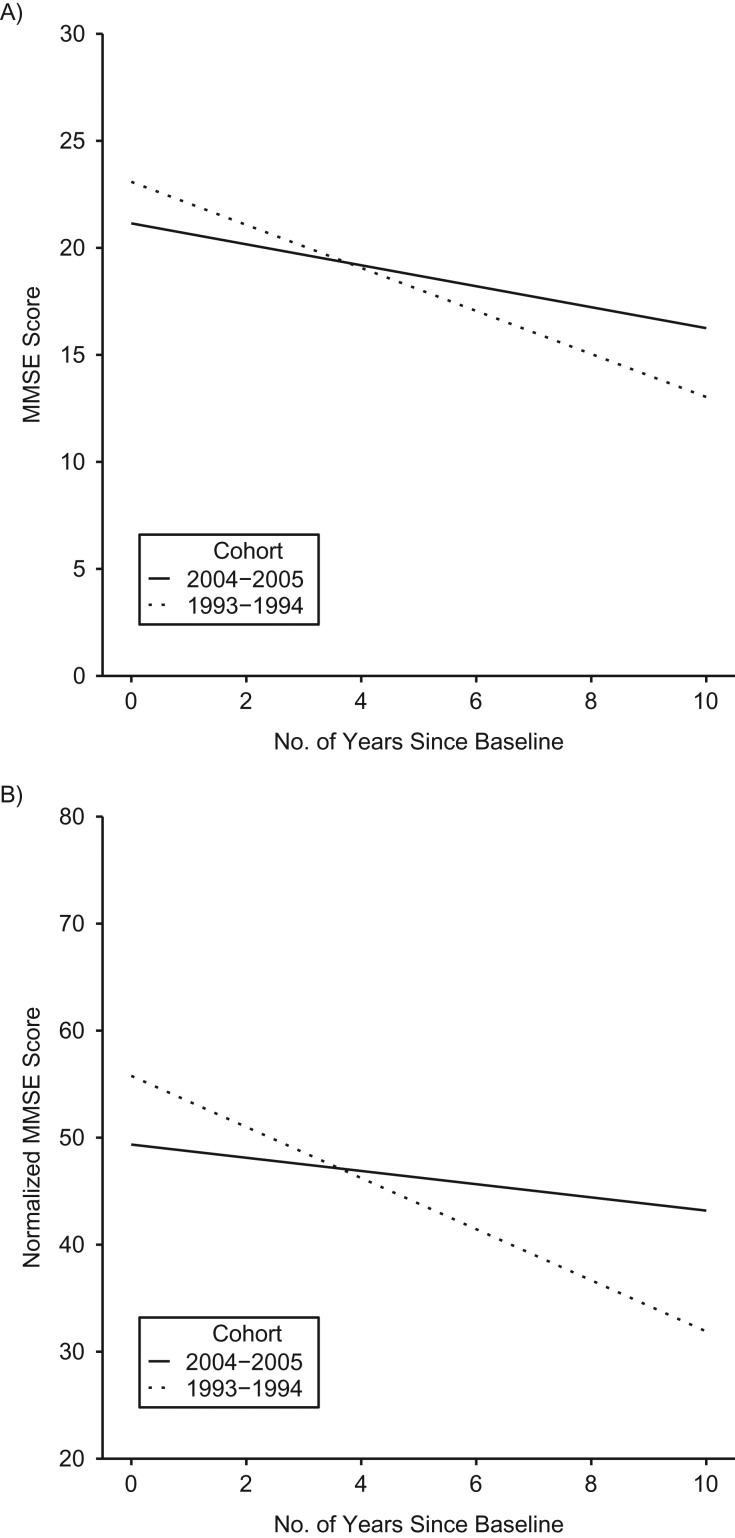
The 2004–2005 cohort showed significantly slower rates of cognitive decline compared with the 1993–1994 cohort (Table 3; Figure 2A). The rate of decline for the 2004–2005 cohort was 0.53 points (95% CI: 0.43, 0.63) per year less compared with the 1993–1994 cohort, controlling for age, sex, and nativity. This finding remained consistent after controlling for years of education (model 2), health characteristics (model 3), and interactions between time and the covariates (model 4). None of the 3-way interaction terms for cohort × time × covariates were statistically significant. The results for the normalized MMSE scores were consistent with the primary analysis (Table 4; Figure 2B).
Table 3.
Differences in Cognitive Decline for Mexican-Americans Aged ≥75 Years (Raw Scores), Hispanic Established Populations for the Epidemiologic Study of the Elderlya, United States, 2004–2005 Cohort Compared With 1993–1994 Cohort
| Variablea | Raw MMSE Scoresb | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| 95% CI | 95% CI | 95% CI | 95% CI | |||||
| 2004–2005 cohort overall | −1.53 | −1.94, −1.22 | −1.94 | −2.33, −1.54 | −2.07 | −2.48, −1.67 | −2.03 | −2.41, 1.66 |
| Time | −1.02 | −1.10, −0.94 | −1.00 | −1.10, −0.90 | −0.98 | −1.08, −0.87 | −0.87 | −1.00, 0.75 |
| Cohort × time | 0.53 | 0.43, 0.63 | 0.52 | 0.41, 0.62 | 0.50 | 0.39, 0.62 | 0.58 | 0.48, 0.67 |
| Age | −0.33 | −0.37, −0.29 | −0.29 | −0.33, −0.25 | −0.29 | −0.33, −0.25 | −0.26 | −0.30, −0.22 |
| Female sex | −0.20 | −0.57, 0.17 | −0.17 | −0.53, 0.18 | −0.003 | −0.37, 0.36 | −0.05 | −0.42, 0.32 |
| US born | 0.84 | 0.47, 1.21 | −0.14 | −0.51, 0.22 | −0.14 | −0.51, 0.22 | −0.20 | −0.58, 0.18 |
| Education | 0.49 | 0.41, 0.62 | 0.47 | 0.42, 0.52 | 0.47 | 0.42, 0.52 | ||
| Diabetes | −0.07 | −0.47, 0.32 | −0.20 | −0.61, 0.22 | ||||
| Heart disease | 0.51 | −0.06, 1.08 | 0.64 | 0.08, 1.20 | ||||
| Hypertension | 0.32 | −0.06, 0.69 | 0.32 | −0.07, 0.71 | ||||
| Stroke | −2.10 | −2.79, −1.41 | −1.92 | −2.62, −1.22 | ||||
| Depression | −1.74 | −2.19, −1.29 | −1.62 | −2.08, −1.16 | ||||
| Covariate × time | ||||||||
| Age | −0.08 | −0.09, −0.07 | ||||||
| Female sex | −0.04 | −0.13, 0.05 | ||||||
| US born | 0.04 | −0.06, 0.13 | ||||||
| Education | −0.02 | −0.03, −0.01 | ||||||
| Diabetes | −0.11 | −0.22, 0.001 | ||||||
| Heart disease | −0.06 | −0.21, 0.09 | ||||||
| Hypertension | −0.07 | −0.16, 0.03 | ||||||
| Stroke | −0.03 | −0.26, 0.19 | ||||||
| Depression | −0.19 | −0.31, 0.07 | ||||||
Abbreviations: CI, confidence interval; MMSE, Mini-Mental State Examination.
a The coefficient for the practice effect term is suppressed from the table.
b Results are from joint longitudinal-survival models. Model 1 controlled for age, sex, and nativity. Model 2 controlled for covariates in model 1 plus education. Model 3 controlled for covariates in model 2 plus diabetes, heart disease, hypertension, stroke, and depression. Model 4 controlled for covariates in model 3 plus interaction terms between time and all covariates.
Figure 2.
Predicted trajectories of raw (A) and normalized (B) Mini-Mental State Examination (MMSE) scores for participants in the 2004–2005 and 1993–1994 cohorts of the Hispanic Established Populations for the Epidemiologic Study of the Elderly, United States. Predicted trajectories are for the reference category: men, age-centered, a dummy variable for practice effects, foreign born, and mean years of education.
Table 4.
Differences in Cognitive Decline Among Mexican-Americans Aged ≥75 Years (Normalized Scores), Hispanic Established Populations for the Epidemiologic Study of the Elderlya, United States, 2004–2005 Cohort Compared With 1993–1994 Cohort
| Variablea | Normalized MMSE Scoresb | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| 95% CI | 95% CI | 95% CI | 95% CI | |||||
| 2004–2005 cohort overall | −4.79 | −6.59, −2.98 | −6.42 | −8.09, −4.74 | −6.43 | −8.12, −4.75 | −6.83 | −8.56, −5.10 |
| Time | −2.39 | −2.69, 2.09 | −2.34 | −2.64, −2.05 | −2.35 | −2.64, 2.05 | −2.21 | −2.67, −1.74 |
| Cohort × time | 1.73 | 1.34, 2.11 | 1.77 | 1.39, 2.15 | 1.79 | 1.41, 2.17 | 1.92 | 1.56, 2.27 |
| Age | −1.35 | −1.52, −1.18 | −1.07 | −1.22, −0.91 | −1.10 | −1.25, −0.95 | −0.95 | −1.13, −0.76 |
| Female | −0.53 | −2.04, 0.99 | −0.46 | −1.84, 0.92 | 0.12 | −1.27, 1.52 | 0.23 | −1.50, 1.96 |
| US born | 3.38 | 1.88, 4.88 | −1.16 | −2.57, 0.25 | −1.24 | −2.64, 0.16 | −1.32 | −3.07, 0.42 |
| Education | 2.27 | 2.09, 2.45 | 2.21 | 2.03, 2.39 | 2.27 | 2.05, 2.50 | ||
| Diabetes | −1.78 | −3.32, −0.23 | −1.34 | −3.24, 0.56 | ||||
| Heart disease | 1.99 | −0.15, 4.13 | 2.47 | −0.12, 5.07 | ||||
| Hypertension | 0.01 | −1.42, 1.45 | 0.37 | −1.43, 2.17 | ||||
| Stroke | −3.58 | −6.17, 1.01 | −4.66 | −7.79, −1.52 | ||||
| Depression | −5.12 | −6.84, 3.41 | −4.96 | −7.05, −2.87 | ||||
| Covariate × time | ||||||||
| Age | −0.15 | −0.20, −0.11 | ||||||
| Female | 0.03 | −0.32, 0, 38 | ||||||
| US born | 0.20 | −0.16, 0.55 | ||||||
| Education | −0.09 | −0.13, 0.05 | ||||||
| Diabetes | −0.29 | −0.69, 0.11 | ||||||
| Heart disease | −0.29 | −0.83, 0.26 | ||||||
| Hypertension | −0.09 | −0.45, 0.27 | ||||||
| Stroke | 0.16 | −0.53, 0.86 | ||||||
| Depression | −0.18 | −0.62, 0.26 | ||||||
Abbreviations: CI, confidence interval; MMSE, Mini-Mental State Examination.
a The coefficient for the practice effect term is suppressed from the table.
b Results are from joint longitudinal-survival models. Model 1 controlled for age, sex, and nativity. Model 2 controlled for covariates in model 1 plus education. Model 3 controlled for covariates in model 2 plus diabetes, heart disease, hypertension, stroke, and depression. Model 4 controlled for covariates in model 3 plus interaction terms between time and all covariates.
Incident cognitive impairment
A total of 496 incident cases of cognitive impairment were identified. This included 317 cases in the 1993–1994 cohort and 179 cases in the 2004–2005 cohort. The overall 10-year incidence rate per 100 person-years was 6.32 (95% CI: 5.79, 6.90). The incidence rate was significantly lower for the 2004–2005 cohort (5.24, 95% CI: 4.51, 6.04) compared with the 1993–1994 cohort (7.18, 95% CI: 6.41, 7.99). The average age for identification of cognitive impairment was 86.6 years for the 2004–2005 cohort and 86.2 years for the 1993–1994 cohort (P = 0.32). The average age of death for participants who became cognitively impaired was 89.8 years for the 2004–2005 cohort and 89.6 years for the 1993–1994 cohort (P = 0.51).
The 2004–2005 cohort had 0.78 (95% CI: 0.65, 0.94) times the risk of incident cognitive impairment as the 1993–1994 cohort (Table 5). After controlling for baseline MMSE score, the 2004–2005 cohort had 0.75 (95% CI: 0.62, 0.90) times lower risk of incident cognitive impairment compared with the 1993–1994 cohort. The lower risk of incident cognitive impairment for the 2004–2005 cohort was attenuated when controlling for years of education but remained statistically significant (hazard ratio = 0.77, 95% CI: 0.64, 0.93). Controlling for baseline health conditions did not substantially change the risk of incident cognitive impairment in the 2004–2005 cohort (hazard ratio = 0.75, 95% CI: 0.62, 0.91).
Table 5.
Hazard Ratios for 10-Year Incident Cognitive Impairment Among Mexican-Americans Aged ≥75 Years, Hispanic Established Populations for the Epidemiologic Study of the Elderlya, United States, 2004–2005 Cohort Compared With 1993–1994 Cohort
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| 2004–2005 cohort overall | 0.78 | 0.65, 0.94 | 0.75 | 0.62, 0.90 | 0.77 | 0.64, 0.93 | 0.75 | 0.62, 0.91 |
| Female sex | 0.98 | 0.82, 1.18 | 1.02 | 0.85, 1.22 | 1.01 | 0.84, 1.22 | 0.99 | 0.82, 1.19 |
| US born | 1.14 | 0.95, 1.37 | 1.22 | 1.01, 1.47 | 1.26 | 1.04, 1.51 | 1.25 | 1.04, 1.51 |
| Baseline MMSE | 0.92 | 0.90, 0.95 | 0.93 | 0.90, 0.96 | 0.93 | 0.90, 0.96 | ||
| Education | 0.98 | 0.95, 1.01 | 0.97 | 0.95, 1.00 | ||||
| Diabetes | 1.20 | 0.98, 1.46 | ||||||
| Heart disease | 1.24 | 0.94, 1.63 | ||||||
| Hypertension | 1.26 | 1.03, 1.53 | ||||||
| Stroke | 1.37 | 0.99, 1.92 | ||||||
| Depression | 1.09 | 0.88, 1.37 | ||||||
Abbreviations: CI, confidence interval; HR, hazard ratio; MMSE, Mini-Mental State Examination.
a Results are from illness-death models for interval-censored data. Model 1 controlled for sex and nativity. Model 2 controlled for covariates in model 1 plus baseline MMSE score. Model 3 controlled for covariates in model 2 plus education. Model 4 controlled for covariates in Model 4 plus diabetes, heart disease, hypertension, stroke, and depression.
DISCUSSION
The present analysis examined cohort differences in the odds of prevalent cognitive impairment, trajectories of cognitive decline, and risk of incident cognitive impairment among older Mexican-Americans. We observed that Mexican-Americans aged ≥75 years in 2004–2005 had approximately 2.5 times higher odds of being cognitively impaired compared with Mexican-Americans aged ≥75 years in 1993–1994. The higher odds of prevalent cognitive impairment for the 2004–2005 cohort were greatest among participants who completed <4 years of education. Conversely, the 2004–2005 cohort exhibited significantly slower rates of cognitive decline and had a significantly lower risk of incident cognitive impairment over a 10-year period.
The prevalence of a disease is based on disease incidence and duration. A potential explanation for the higher prevalence but lower incidence of cognitive impairment in the 2004–2005 cohort is that this cohort might be living longer with cognitive impairment. However, the illness-death model indicated that the risk of transitioning from cognitively impaired to deceased was not significantly different between the 2 cohorts. Prior studies have not investigated cohort differences in survival following the onset of cognitive impairment in the H-EPESE. In our sample, the average time between age of first being observed to be cognitively impaired and death was 3.20 years for the 2004–2005 cohort and 3.38 years for the 1993–1994 cohort. A recent analysis of data from the Framingham Heart Study revealed that survival after a diagnosis of dementia had decreased from an average of 6 years in 1977–1984 to 3 years in 2004–2008 (57). A compression of morbidity for cognitive impairment has also be observed in the Health and Retirement Study (58).
The decreasing trends for ADRD prevalence and incidence among recent cohorts of older adults have been attributed to increased educational attainment (13). We observed that the 2004–2005 cohort had significantly higher odds of prevalent cognitive impairment compared with the 1993–1994 cohort despite the 2004–2005 cohort’s having completed nearly 1 year of education more on average. The decreased risk of incident cognitive impairment remained statistically significant after controlling for education. A possible explanation for this finding is that increases in education have not coincided with improvements in educational quality. Past research indicates that reading ability and other measures that approximate educational quality are stronger predictors of cognitive functioning than educational attainment, especially for minority older adults (59, 60). Educational quality is also important to consider given that a significantly higher percentage of participants in the 2004–2005 cohort were born in the United States compared with the 1993–1994 cohort. The United States and Mexico have substantially different educational systems, which could influence an individual’s risk of cognitive impairment through disparities in educational quality.
We observed that US-born participants had significantly higher risk of incident cognitive impairment compared with foreign-born participants. However, a term for interaction between cohort and nativity was not statistically significant. Prior research indicates that nativity differences in cognitive functioning are influenced by sex and, for foreign-born Mexican-Americans, the age at which an individual migrated to the United States. Foreign-born Mexican-American men who migrated to the United States as middle-aged adults show slower rates of cognitive decline (61) and have a lower risk of cognitive impairment (62) compared with US-born Mexican-American men. However, foreign-born Hispanics regardless of sex have been observed to live longer with cognitive impairment than US-born Hispanics (31, 63).
The 2004–2005 cohort also had significantly higher prevalence of diabetes and hypertension. Diabetes and midlife hypertension are important risk factors for ADRD (64). However, controlling for health conditions explained very little of the decreased risk of incident cognitive impairment in the 2004–2005 cohort. Prior studies have reported that health characteristics explained only a small amount of the decrease in ADRD incidence (2, 9), although reducing the prevalence of chronic health conditions and increased engagement in positive health behaviors might have a substantial impact on the prevalence of ADRD (64).
This analysis has important limitations. First, the MMSE is the only measure of cognitive functioning in the H-EPESE. The MMSE was originally designed to be used in clinical settings as a screening tool for ADRD and severe cognitive impairment (39). The MMSE has limited accuracy for detecting ADRD in community settings (65), especially among older adults with low educational attainment (65) or whose first language is not English (66). Consequently, some H-EPESE participants might have been incorrectly classified as cognitively impaired or cognitively intact. We also did not consider functional limitations, which must be present to warrant a clinical diagnosis ADRD (67), in our definition of cognitive impairment. Comparing trends reported in different studies is complicated by the fact that diagnostic criteria for ADRD vary across studies, and this can dramatically decrease or increase estimates (68, 69). Our estimates for the prevalence and incidence of cognitive impairment would have been lower had participants been required to be functionally impaired as well.
A second limitation is that the MMSE has poor psychometric properties for detecting changes in cognitive functioning (39, 70). We analyzed trajectories of cognitive decline using the raw MMSE scores and using a normalized transformation of the MMSE to account for floor/ceiling effects. While these analyses produced consistent results, our findings need to be replicated using data for older Mexican-Americans that include a comprehensive cognitive evaluation. The findings for the trajectories of cognitive decline might have also been influenced by the lower percentage of proxy interviews in the 2004–2005 cohort compared with the 1993–1994 cohort. An additional limitation is that the H-EPESE does not include information for potentially important risk factors such as level of physical activity or midlife health conditions. We also did not control for other measures of socioeconomic status, such as income. However, it is unlikely that controlling for income would have substantially changed our results given that approximately 75% of participants in both cohorts reported having a yearly household income of less than $15,000. Finally, it is important to consider the representativeness of the sample populations with respect to the general population of Mexican-Americans aged ≥75 years living in the Southwestern United States. The H-EPESE sampling procedures were designed so that participants in both cohorts were representative of older Mexican-Americans living in the Southwestern United States during the 1990s and 2000s. The 2004–2005 cohort had higher educational attainment than the 1993–1994 cohort, and educational attainment has continued to increase among older Hispanics since 2005 (33). The prevalence of chronic health conditions associated with greater risk of cognitive impairment has also increased among Hispanics (71). Population-level changes of risk factors for cognitive impairment make it important to continue monitoring trends in ADRD and cognitive impairment prevalence and incidence among older Mexican-Americans.
To summarize, this analysis detected significant cohort differences in the odds of prevalent cognitive impairment, risk of incident cognitive impairment, and rates of cognitive decline among Mexican-Americans aged ≥75 years. Future research is needed to identify potentially modifiable environmental, social, neighborhood, health, and lifestyle characteristics that contribute to cohort differences in cognitive impairment and cognitive decline among older Mexican-Americans.
ACKNOWLEDGMENTS
Author affiliations: Division of Rehabilitation Sciences, School of Health Professions, University of Texas Medical Branch, Galveston, Texas (Brian Downer); Department of Sociology, University of Nebraska, Lincoln, Lincoln, Nebraska (Marc A. Garcia); Institute for Ethnic Studies, University of Nebraska, Lincoln, Lincoln, Nebraska (Marc A. Garcia); Division of Geriatrics, Department of Internal Medicine, University of Texas Medical Branch, Galveston, Texas (Mukaila Raji); and Preventive Medicine and Community Health, University of Texas Medical Branch, Galveston, Texas (Kyriakos S. Markides).
This work was supported by the National Institute on Aging (National Institutes of Health) (grants 5R01AG010939-24, 2T32AG00027018-A1, and 5P30AG024832-14).
The results of this manuscript were presented at the American Association for Geriatric Psychiatry 2018 Annual Meeting, March 15–18, 2018, Honolulu, Hawaii.
The study sponsors had no role in the study design, interpretation of results, or writing of the manuscript. The authors are solely responsible for the content and the writing of the manuscript.
Conflict of interest: none declared.
Abbreviations
- ADRD
Alzheimer disease and related dementias
- CI
confidence interval
- H-EPESE
Hispanic Established Populations for the Epidemiologic Study of the Elderly
- MMSE
Mini-Mental State Examination
REFERENCES
- 1. Hebert LE, Weuve J, Scherr PA, et al. . Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Satizabal CL, Beiser AS, Chouraki V, et al. . Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374(6):523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Langa KM, Larson EB, Crimmins EM, et al. . A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177(1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rocca WA, Petersen RC, Knopman DS, et al. . Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7(1):80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schrijvers EM, Verhaaren BF, Koudstaal PJ, et al. . Is dementia incidence declining? Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology. 2012;78(19):1456–1463. [DOI] [PubMed] [Google Scholar]
- 6. Skoog I, Börjesson-Hanson A, Kern S, et al. . Decreasing prevalence of dementia in 85-year olds examined 22 years apart: the influence of education and stroke. Sci Rep. 2017;7:6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cerasuolo JO, Cipriano LE, Sposato LA, et al. . Population-based stroke and dementia incidence trends: age and sex variations. Alzheimers Dement. 2017;13(10):1081–1088. [DOI] [PubMed] [Google Scholar]
- 8. Crimmins EM, Saito Y, Kim JK. Change in cognitively healthy and cognitively impaired life expectancy in the United States: 2000–2010. SSM Popul Health. 2016;2:793–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Langa KM, Larson EB, Karlawish JH, et al. . Trends in the prevalence and mortality of cognitive impairment in the United States: is there evidence of a compression of cognitive morbidity? Alzheimers Dement. 2008;4(2):134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prince M, Ali GC, Guerchet M, et al. . Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther. 2016;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hudomiet P, Hurd MD, Rohwedder S. Dementia prevalence in the United States in 2000 and 2012: estimates based on a nationally representative study. J Gerontol B Psychol Sci Soc Sci. 2018;73(suppl 1):S10–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weden MM, Shih RA, Kabeto MU, et al. . Secular trends in dementia and cognitive impairment of US rural and urban older adults. Am J Prev Med. 2018;54(2):164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu YT, Beiser AS, Breteler MMB, et al. . The changing prevalence and incidence of dementia over time—current evidence. Nat Rev Neurol. 2017;13(6):327–339. [DOI] [PubMed] [Google Scholar]
- 14. Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. N Engl J Med. 2013;369(24):2275–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohara T, Hata J, Yoshida D, et al. . Trends in dementia prevalence, incidence, and survival rate in a Japanese community. Neurology. 2017;88(20):1925–1932. [DOI] [PubMed] [Google Scholar]
- 16. Hebert LE, Bienias JL, Aggarwal NT, et al. . Change in risk of Alzheimer disease over time. Neurology. 2010;75(9):786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rocca WA, Cha RH, Waring SC, et al. . Incidence of dementia and Alzheimer’s disease: a reanalysis of data from Rochester, Minnesota, 1975–1984. Am J Epidemiol. 1998;148(1):51–62. [DOI] [PubMed] [Google Scholar]
- 18. Choi H, Schoeni RF, Martin LG, et al. . Trends in the prevalence and disparity in cognitive limitations of Americans 55–69 years old. J Gerontol B Psychol Sci Soc Sci. 2018;73(suppl 1):S29–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weuve J, Rajan KB, Barnes LL, et al. . Secular trends in cognitive performance in older black and white US adults, 1993–2012: findings from the Chicago Health and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2018;73(suppl 1):S73–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van den Kommer TN, Deeg DJH, van der Flier WM, et al. . Time trend in persistent cognitive decline: results from the Longitudinal Aging Study Amsterdam. J Gerontol B Psychol Sci Soc Sci. 2018;73(suppl 1):S57–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yaffe K, Falvey C, Harris TB, et al. . Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. 2013;347:f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gurland BJ, Wilder DE, Lantigua R, et al. . Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry. 1999;14(6):481–493. [PubMed] [Google Scholar]
- 23. Tang MX, Cross P, Andrews H, et al. . Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49–56. [DOI] [PubMed] [Google Scholar]
- 24. Mayeda ER, Karter AJ, Huang ES, et al. . Racial/ethnic differences in dementia risk among older type 2 diabetic patients: the diabetes and aging study. Diabetes Care. 2014;37(4):1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garcia MA, Saenz J, Downer B, et al. . The role of education in the association between race/ethnicity/nativity, cognitive impairment, and dementia among older adults in the United States. Demogr Res. 2018;38:155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gao S, Ogunniyi A, Hall KS, et al. . Dementia incidence declined in African-Americans but not in Yoruba. Alzheimers Dement. 2016;12(3):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Markides KS, Gerst K. Immigration, aging, and health in the United States In: Settersten RA, Angel JL, eds. Handbook of Sociology of Aging. New York, NY: Springer; 2011:103–116. [Google Scholar]
- 28. Noble JM, Schupf N, Manly JJ, et al. . Secular trends in the incidence of dementia in a multi-ethnic community. J Alzheimers Dis. 2017;60(3):1065–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sheffield KM, Peek MK. Changes in the prevalence of cognitive impairment among older Americans, 1993–2004: overall trends and differences by race/ethnicity. Am J Epidemiol. 2011;174(3):274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ennis SR, Ríos-Vargas M, Albert NG. The Hispanic Population: 2010 Washington, DC: United States Census Bureau; 2011. http://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf. Accessed January 22, 2018.
- 31. Garcia MA, Downer B, Chiu CT, et al. . Racial/ethnic and nativity differences in cognitive life expectancies among older adults in the United States [published online ahead of print September 16, 2017]. Gerontologist. (doi: 10.1093/geront/gnx142). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Centers for Disease Control and Prevention National Diabetes Statistics Report, 2017 Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed January 16, 2018.
- 33. United States Census Bureau Table 1. Educational attainment of the population 18 years and over, by age, sex, race, and Hispanic origin: 2014. Washington, DC: United States Census Bureau; 2014. https://www.census.gov/data/tables/2014/demo/educational-attainment/cps-detailed-tables.html. Accessed January 15, 2018.
- 34. Markides KS, Rudkin L, Angel RJ, et al. . Health status of hispanic elderly In: Martin LG, Soldo BJ, eds. Racial and Ethnic Differences in the Health of Older Americans. Washington, DC: National Academy Press; 1997:285–300. [PubMed] [Google Scholar]
- 35. Eschbach K, Ostir GV, Patel KV, et al. . Neighborhood context and mortality among older Mexican Americans: is there a barrio advantage? Am J Public Health. 2004;94(10):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Markides KS. Hispanic established populations for the epidemiologic studies of the elderly, 1993–1994: (Arizona, California, Colorado, New Mexico, and Texas) Ann Arbor, MI: Inter-university Consortium for Political and Social Research; 2009. 10.3886/ICPSR02851.v2. Accessed June 26, 2018. [DOI]
- 37. Markides KS, Ray LA, Angel R, et al. . Hispanic Established Populations for the Epidemiologic Study of the Elderly (HEPESE) Wave 5, 2004–2005 (Arizona, California, Colorado, New Mexico, and Texas) Ann Arbor, MI: Inter-university Consortium for Political and Social Research; 2009. 10.3886/ICPSR25041.v1. Accessed June 6, 2018. [DOI]
- 38. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 39. Tombaugh TN, McIntyre NJ. The Mini-Mental State Examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935. [DOI] [PubMed] [Google Scholar]
- 40. Proust-Lima C, Amieva H, Dartigues JF, et al. . Sensitivity of four psychometric tests to measure cognitive changes in brain aging-population-based studies. Am J Epidemiol. 2007;165(3):344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Philipps V, Amieva H, Andrieu S, et al. . Normalized Mini-Mental State Examination for assessing cognitive change in population-based brain aging studies. Neuroepidemiology. 2014;43(1):15–25. [DOI] [PubMed] [Google Scholar]
- 42. Proust C, Jacqmin-Gadda H, Taylor JM, et al. . A nonlinear model with latent process for cognitive evolution using multivariate longitudinal data. Biometrics. 2006;62(4):1014–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Proust-Lima C, Dartigues JF, Jacqmin-Gadda H. Misuse of the linear mixed model when evaluating risk factors of cognitive decline. Am J Epidemiol. 2011;174(9):1077–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Proust-Lima C, Philipps V. NormPsy: normalization of psychometric tests. R package version 1.0.3 Bordeaux, France: Bordeaux Population Health; 2015. https://CRAN.R-project.org/package=NormPsy. Accessed August 22, 2018.
- 45. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 46. Guo X, Carlin BP. Separate and joint modeling of longitudinal and event time data using standard computer packages. Am Stat. 2004;58(1):16–24. [Google Scholar]
- 47. Wilson RS, Beckett LA, Bienias JL, et al. . Terminal decline in cognitive function. Neurology. 2003;60(11):1782–1787. [DOI] [PubMed] [Google Scholar]
- 48. Wilson RS, Beck TL, Bienias JL, et al. . Terminal cognitive decline: accelerated loss of cognition in the last years of life. Psychosom Med. 2007;69(2):131–137. [DOI] [PubMed] [Google Scholar]
- 49. Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 50. Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16(20):2349–2380. [DOI] [PubMed] [Google Scholar]
- 51. Vivot A, Power MC, Glymour MM, et al. . Jump, hop, or skip: modeling practice effects in studies of determinants of cognitive change in older adults. Am J Epidemiol. 2016;183(4):302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rizopoulos D. JM: an R package for the joint modelling of longitudinal and time-to-event data. J Stat Softw. 2010;35(9):1–33.21603108 [Google Scholar]
- 53. Leffondré K, Touraine C, Helmer C, et al. . Interval-censored time-to-event and competing risk with death: is the illness-death model more accurate than the Cox model? Int J Epidemiol. 2013;42(4):1177–1186. [DOI] [PubMed] [Google Scholar]
- 54. Joly P, Commenges D, Helmer C, et al. . A penalized likelihood approach for an illness-death model with interval-censored data: application to age-specific incidence of dementia. Biostatistics. 2002;3(3):433–443. [DOI] [PubMed] [Google Scholar]
- 55. Touraine C, Joly P, Gerds TA. SmoothHazard: Fitting illness-death model for interval-censored data. R package version 1.2.3, 2014.
- 56. R Development Core Team R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; 2011.
- 57. Dufouil C, Beiser A, Chêne G, et al. . Are trends in dementia incidence associated with compression in morbidity? Evidence from the Framingham Heart Study. J Gerontol B Psychol Sci Soc Sci. 2018;73(suppl 1):S65–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Crimmins EM, Saito Y, Kim JK, et al. . Educational differences in the prevalence of dementia and life expectancy with dementia: changes from 2000 to 2010. J Gerontol B Psychol Sci Soc Sci. 2018;73(suppl 1):S20–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Manly JJ, Touradji P, Tang MX, et al. . Literacy and memory decline among ethnically diverse elders. J Clin Exp Neuropsychol. 2003;25(5):680–690. [DOI] [PubMed] [Google Scholar]
- 60. Dotson VM, Kitner-Triolo MH, Evans MK, et al. . Effects of race and socioeconomic status on the relative influence of education and literacy on cognitive functioning. J Int Neuropsychol Soc. 2009;15(4):580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hill TD, Angel JL, Balistreri KS, et al. . Immigrant status and cognitive functioning in late-life: an examination of gender variations in the healthy immigrant effect. Soc Sci Med. 2012;75(12):2076–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Garcia MA, Reyes AM, Downer B, et al. . Age of migration and the incidence of cognitive impairment: a cohort study of Elder Mexican-Americans. Innovation Aging. 2017;1(3):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Garcia MA, Saenz JL, Downer B, et al. . Age of migration differentials in life expectancy with cognitive impairment: 20-year findings from the Hispanic-EPESE. Gerontologist. 2018;58(5):894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Norton S, Matthews FE, Barnes DE, et al. . Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. [DOI] [PubMed] [Google Scholar]
- 65. Mitchell AJ. A meta-analysis of the accuracy of the Mini-Mental State Examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. 2009;43(4):411–431. [DOI] [PubMed] [Google Scholar]
- 66. Ramirez M, Teresi JA, Holmes D, et al. . Differential item functioning (DIF) and the Mini-Mental State Examination (MMSE). Overview, sample, and issues of translation. Med Care. 2006;44(11 suppl 3):S95–S106. [DOI] [PubMed] [Google Scholar]
- 67. McKhann GM, Knopman DS, Chertkow H, et al. . The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wilson RS, Weir DR, Leurgans SE, et al. . Sources of variability in estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimers Dement. 2011;7(1):74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Erkinjuntti T, Ostbye T, Steenhuis R, et al. . The effect of different diagnostic criteria on the prevalence of dementia. N Engl J Med. 1997;337(23):1667–1674. [DOI] [PubMed] [Google Scholar]
- 70. Spencer RJ, Wendell CR, Giggey PP, et al. . Psychometric limitations of the Mini-Mental State Examination among nondemented older adults: an evaluation of neurocognitive and magnetic resonance imaging correlates. Exp Aging Res. 2013;39(4):382–397. [DOI] [PubMed] [Google Scholar]
- 71. Menke A, Casagrande S, Geiss L, et al. . Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–1029. [DOI] [PubMed] [Google Scholar]