Figure 1.
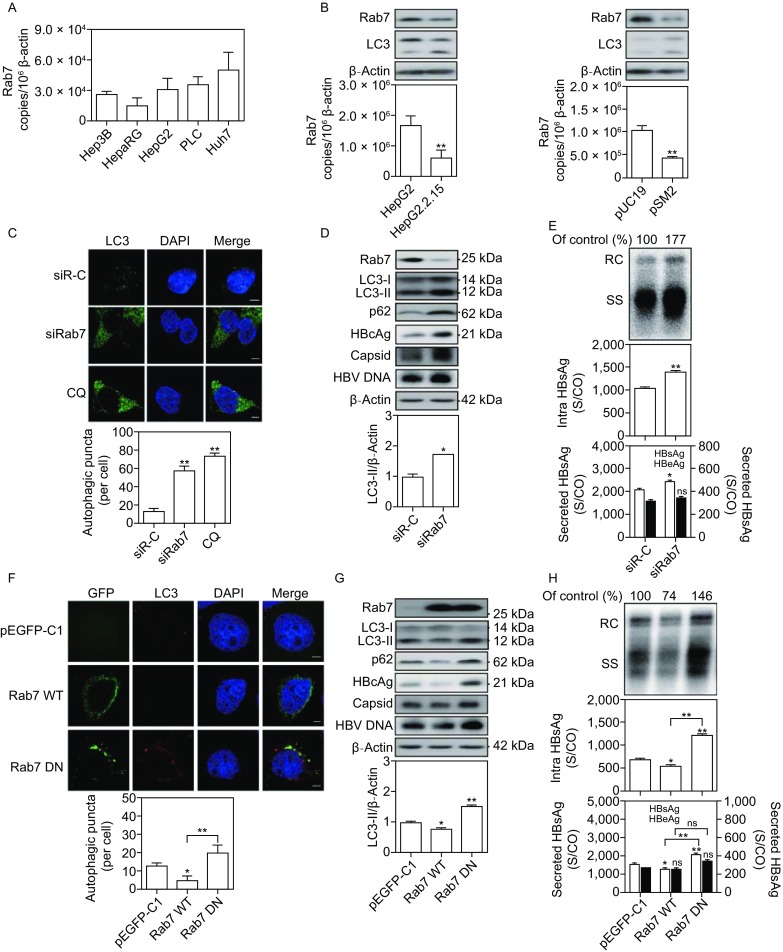
Rab7 silencing promotes HBV replication and HBsAg production, but Rab7 activation decreases. (A) The mRNA levels of Rab7 in different hepatoma cell lines, including Hep3B, HepaRG, HepG2, PLC and Huh7 cells, were determined by real-time (RT)-PCR using specific primers. (B) The protein and mRNA levels of Rab7 in HepG2 and HepG2.2.15 cells as well as in Huh7 cells after transfection with HBV plasmid pSM2 or control vector pUC19 were determined by Western blot and real-time RT-PCR, respectively. (C and D) HepG2.2.15 cells were transfected with 20 nmol/L siRNAs against Rab7 (siRab7) or control siRNA (siR-C). After 48 h, the transfected cells were fixed, incubated with primary antibody anti-LC3 and stained with Alexa Fluor 594-conjugated anti-rabbit secondary antibody IgG. The transfected cells were imaged by confocal microscopy. The cells were treated with 10 µmol/L CQ for 24 h as a positive control. Rab7, LC3, p62 and HBcAg expression and viral nucleocapsid levels were analyzed by Western blot. Detection of encapsidated HBV DNA was performed by Southern blot. (E) Huh7 cells were cotransfected with the pSM2 plasmid and siRab7 or siR-C at 20 nmol/L and harvested after 72 h. Analysis of secreted HBsAg and HBeAg in culture supernatants and intracellular HBsAg from cell lysates was performed by a chemiluminescent microparticle immunoassay (CMIA). Analyses of HBV replicative intermediates inside the cells were performed by Southern blot. (F) Huh7 cells were transfected with an expression vector carrying wild-type Rab7 (Rab7 WT), dominant negative Rab7 (Rab7 DN), or a control vector pEGFP-C1. After 48 h, the cells were imaged using a confocal microscopy. (G and H) Huh7 cells were cotransfected with pSM2 plasmid and Rab7 WT, Rab7 DN, or vector pEGFP-C1, and harvested after 72 h. S/CO: signal to cutoff ratio; RC: relaxed circular DNA; SS: single-stranded DNA. The data are shown as mean ± SEM. *P < 0.05; **P < 0.01; ns, not significant
