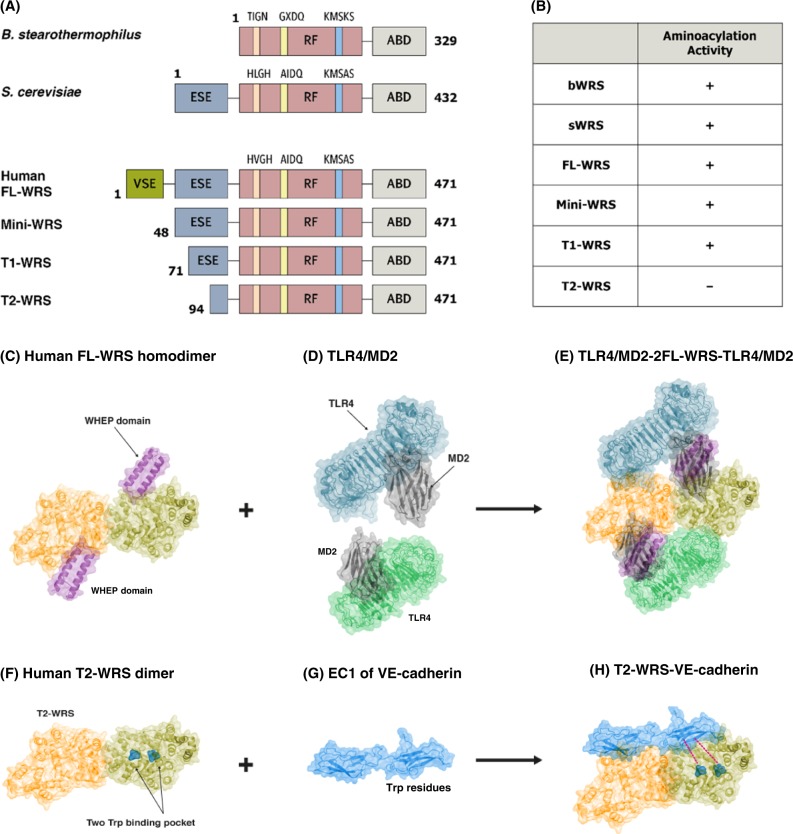
Fig. 2. Various structures of WRS and their interactions with specific receptors.
a Schematic representation of domains in WRS from prokaryotes (B. stearothermophilus), lower eukaryotes (S. cerevisiae), and higher organisms (H. sapiens). The Rossmann Fold (RF) catalytic domain and anticodon-binding domain (ABD) are well conserved in all WRSs. In the RF domain, the three characteristic types of motif have slightly different sequences among species. The eukaryotic-specific extension (ESE) is common in eukaryotic WRSs. Human full-length (FL)-WRS also has the vertebrate-specific extension (VSE) at the N-terminal appended site. Alternative splicing can produce a mini-WRS where a portion of the VSE is truncated. After being secreted into the extracellular space, the T1 and T2-WRSs are generated by proteolytic cleavage, which removes the ESE, including the entire VSE sequence. b The aminoacylation activity of the various forms of WRSs in different species. All the WRSs, with the exception of T2-WRS, are capable of aminoacylation. c–h Representative model of the structural arrangement of WRS and their specific interactions with receptors. FL-WRS (c), dimeric human FL-WRS (PDB 1R6T) binding to two TLR4/MD2 heterodimers (PDB 3FXI) through the WHEP domain and the N154 terminus in trans (d, e), T2-WRS (PDB 1O5T) with truncated WHEP entirely cuts off the N-terminus (f), interaction of T2-WRS with the EC1 domain in VE-cadherin (PDB 3PPE). Trp residues present in the EC1 domain bind to the Trp binding pocket present in the RF domain of WRS (g, h)