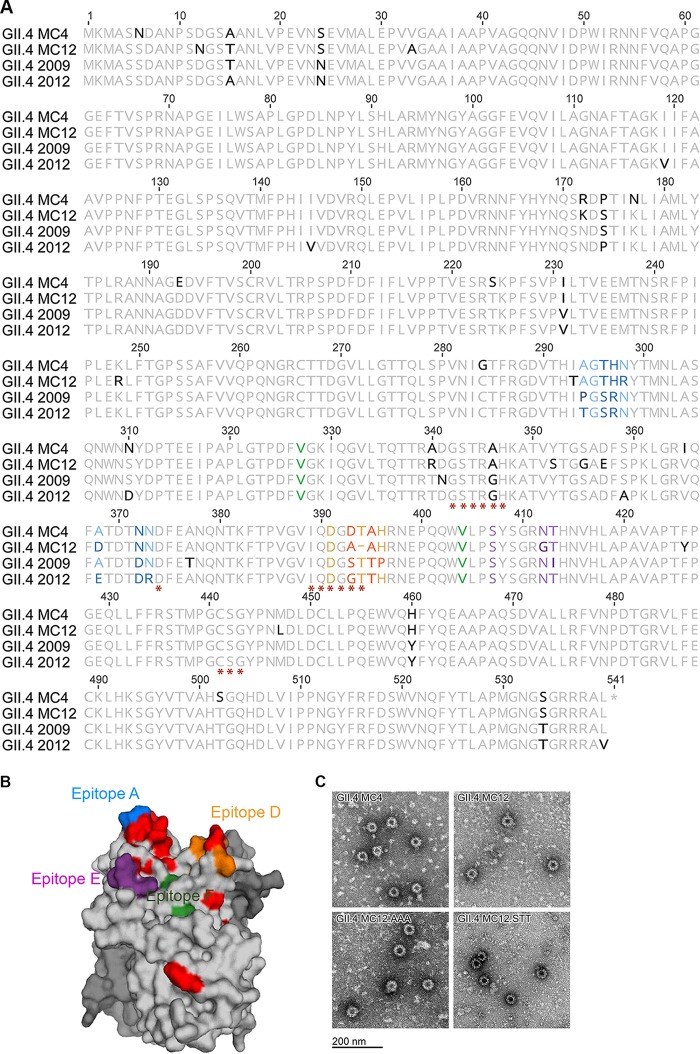
FIG 2.
GII.4 MC4 and MC12 capsid protein. (A) Capsid protein amino acid sequences of GII.4 MC4 and MC12 compared to GII.4 2009 New Orleans and 2012 Sydney. Residues known to interact with carbohydrate ligand (*) and antibodies that block VLP-ligand interaction (epitopes A [blue], D [orange], E [purple], and F [green]) are indicated. Residues that vary between the compared sequences are in boldface. (B) Side view of GII.4 MC12 P domain dimer homology model based on GII.4 2012 with one chain color-coded by epitopes as in panel A. The carbohydrate binding domain is not visible in this orientation. Residues that vary in MC12 compared to the consensus sequence of GII.4 2009 and 2012 are colored red. (C) Electron micrograph of VLP created for this study.