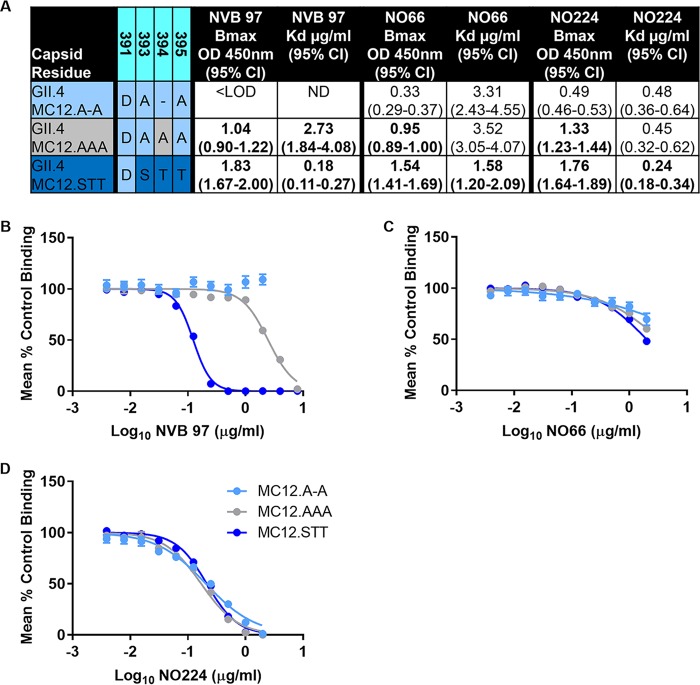
FIG 5.
The number and identity of epitope D residues impacts VLP interaction with blockade antibodies by multiple mechanisms. (A) GII.4 MC12, MC12.AAA, and MC12.STT binding to MAbs NVB 97, NO66, and NO224 was analyzed by single-site binding curve fitting, and maximum binding (Bmax) and dissociation constants (Kd) were calculated to differentiate the effects of sequence changes on antibody-epitope access and binding strength, respectively. Boldface numbers are significantly different from GII.4 MC12.A-A. (B to D) Antibody function, as measured by blockade of ligand binding potency, the IC50 titer, was determined by dose-response nonlinear curve fit analysis of the mean percent control binding with 95% CI values (error bars).