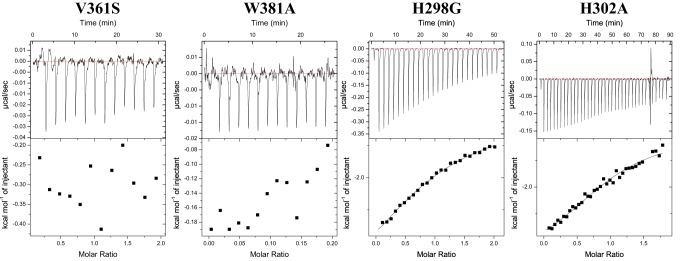
FIG 9.
Thermodynamic properties of GCDCA binding to GII.10 P domain mutants. Titrations were performed at 25°C by injecting consecutive aliquots of 450 μM GCDCA into 45 μM GII.10 P domain mutants (i.e., H298G, R299A, H302A, V361S, and W381A). Substitutions of V361S and W381A led to a complete loss of GCDCA binding. Substitutions of H298A and H302G led to severalfold affinity reductions, with Kd values of 18 μM and 107 μM, respectively. The R299A mutant did not influence the binding affinity.