Figure 2. Peroxisome fission in the growth and division model.
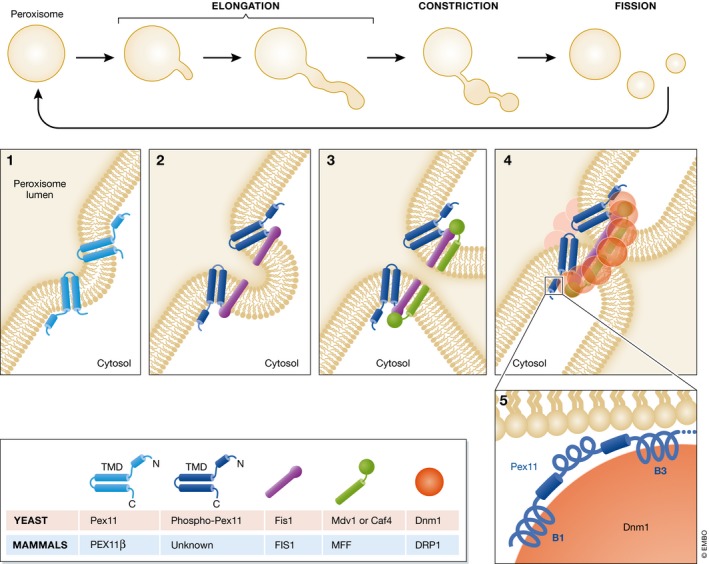
According to the growth and division model, peroxisome fission happens in a 3‐step process. During the first step of elongation, Pex11 (PEX11β in mammals), a transmembrane protein that imparts curvature to peroxisome membranes (panel 1), is essential for the elongation step. The topology shown here for Pex11 is based on studies in H. polymorpha 67. The second step, involving membrane constriction, is poorly understood and we do not know any proteins implicated in this step. The third step, peroxisome fission, starts in P. pastoris with the phosphorylation of Pex11(S173) that stimulates its interaction with the adaptor, Fis1 (panel 2) 78. Note that the topology of PpPex11 has not been documented, so it is unclear whether the phosphorylation is on the cytosolic or the peroxisome matrix side. Fis1 then recruits the peripheral receptors, Mdv1 and/or Caf4 (panel 3) 75. Mdv1 and/or Caf4 assemble a Dnm1 ring around the peroxisome constriction site (panel 4). Mammals do not have homologues for these proteins, and DRP1 is recruited to peroxisomes by MFF and FIS1 79. Yeast Dnm1 interacts with Fis1 and two Pex11 helices named B1 and B3 (panel 5). The hydrolysis of GTP by Dnm1, enhanced by the interaction with the B3 helix of Pex11, leads to a constriction that divides the peroxisome 82.
