Abstract
The National Cancer Institute’s (NCI) Radiation Research Program (RRP) is endeavoring to increase the relevance of preclinical research to improve outcomes of radiation therapy for cancer patients. These efforts include conducting symposia, workshops and educational sessions at annual meetings of professional societies, including the American Association of Physicists in Medicine, American Society of Radiation Oncology, Radiation Research Society (RRS), Radiosurgery Society, Society of Nuclear Medicine and Molecular Imaging, Society for Immunotherapy of Cancer and the American Association of Immunology. A symposium entitled “Radiation-Drug Combinations to Improve Clinical Outcomes and Reduce Normal Tissue Toxicities” was conducted by the NCI’s RRP during the 63rd Annual Meeting of the RRS on October 16, 2017 in Cancun, Mexico. In this symposium, discussions were held to address the challenges in developing radiation-drug combinations, optimal approaches with scientific evidence to replace standard-of-care, approaches to reduce normal tissue toxicities and enhance post-treatment quality-of-life and recent advances in antibody-drug conjugates. The symposium included two broad overview talks followed by two talks illustrating examples of radiation-drug combinations under development. The overview talks identified the essential preclinical infrastructure necessary to accelerate progress in the development of evidence and important challenges in the translation of drug combinations to the clinic from the laboratory. Also addressed, in the example talks (in light of the suggested guidelines and identified challenges), were the development and translation of novel antibody drug conjugates as well as repurposing of drugs to improve efficacy and reduce normal tissue toxicities. Participation among a cross section of clinicians, scientists and scholars-in-training alike who work in this focused area highlighted the importance of continued discussions to identify and address complex challenges in this emerging area in radiation oncology.
INTRODUCTION
Over 50% of cancer patients receive radiotherapy, which has been successfully combined with other treatment modalities (1–3). Approximately 40% of curative cancer treatment involves radiation either as a monotherapy or in combination with other modalities (4, 5). Further therapeutic benefit in the use of radiotherapy is expected to come from: 1. particle therapy (protons and carbon); 2. improvements in radiation dose delivery to the tumor; 3. biomarker-driven risk stratification of patients or patient cohorts, by which radiation dose is selected; and 4. rational integration of radiation-drug combinations. Notably, all of these advances can be synergistic with one another (1). Since the 1980s, it has been clearly demonstrated that combined radio- and chemotherapy improve tumor control and patient survival (2). This clinically successful paradigm has for the most part exclusively utilized cytotoxic chemotherapeutic drugs such as taxanes, platinums, gemcitabine, 5-fluorouracil and temozolamide, and continues as the standard-of-care in the management of many locally advanced, nonmetastatic solid tumors (2). While some may argue that these drugs are “targeted”, well-established nonmolecular-targeted, their side effect profile is broad and precludes any further dose intensification in combination with radiation.
There has been a tremendous growth in knowledge of the molecular basis of oncogenesis over the last two decades, and drugs targeting tumor-specific pathways have been translated to the clinic. Shockingly, only one targeted drug, cetuximab, has demonstrated clinical efficacy in combination with radiotherapy (3). Cetuximab is an antibody blocking the epidermal growth factor receptor (EGFR) and inhibits pro-survival signaling in tumors (6, 7). In a phase III trial, cetuximab was shown to increase cancer cure rates in combination with radiotherapy compared to radiotherapy alone for head and neck squamous cell cancer (HNSCC) (6), although only modest improvements in short-term survival were observed when the drug was combined with chemotherapy (2, 6, 8). Disappointingly, the success of cetuximab combined with radiation has not been replicated with other targeted agents, and in fact, few such combinations have been tested in the phase III setting. Thus, cetuximab with radiation treatment failed to qualify as standard-of-care. Therefore, there remains a tremendous need to develop more biologically targeted drug-radiotherapy combinations as treatments become increasingly customized to patient cohorts or individual patient bio-marker profiles (9).
There continues to be improved understanding of cancer biology and radiation response including gene expression and epigenetic alterations, cellular signaling, protein posttranslational modifications and differences in DNA damage response and repair between tumor and normal tissue (9). These developments open many new critical molecular pathways that can be pharmacologically targeted and exploited in conjunction with radiotherapy. Such radiation-drug combination strategies may include the use of: DNA damage response inhibitors, inhibitors of pro-survival signaling pathways, hypoxic cell sensitizers, metabolic inhibitors, immune modulators, growth factor inhibitors, anti-invasive drugs and anti-angiogenic agents (5).
A collaborative effort among members of the Translational Research Program of the Radiation Oncology Therapy Group (formerly RTOG, now part of NRG4) and the National Cancer Institute (NCI) was established in 2012 to identify and address research, development and translational challenges relevant to radiation-drug combinations (2). Development of radiation-drug combinations presents many challenges, which include: 1. limited relevance of preclinical studies; 2. a general lack of enthusiasm by pharmaceutical industries; 3. few individuals with the necessary skill set and institutional commitment to ensure a successful research program (2); and 4. a lack of rational and standardized approaches to develop and translate radiation-drug combinations (5).
All currently used clinical radiosensitizer drugs were originally developed as cancer monotherapies, followed by testing in combination with radiotherapy. There is a strong need for more relevant preclinical studies with mechanistic and rational underpinnings for developing more effective radiation-drug combinations (10). These include: identifying and validating appropriate targets for combining with radiation; studying reproductive/clonogenic cell death; selecting more appropriate preclinical model systems; translating dose-schedule regimen from laboratory to clinic; and optimizing and demonstrating safety and efficacy in applicable preclinical tumor models. To ensure high-quality studies, reproducibility and translatability of preclinical studies are equally critical, requiring more robust experimental design, execution and reporting of laboratory studies of targeted agents for clinical use with radiation (10, 11). Finally, preclinical studies need to evaluate experimental radiosensitizers in the context of clinically used cytotoxic chemotherapies, as they remain the gold standard for patient care.
A key impediment responsible for the limited success in translating radiation-drug combinations to the clinic has been a lack of focused collaboration among academic basic science laboratories, pharmaceutical companies and clinicians, as well as the limited availability of financial support for such ventures (2, 12). The RRP has been encouraging small business and academic partnerships by advising the Small Business Innovation Research (SBIR) Development Center at the NCI of priorities in radiation oncology research and helping to oversee awards, leading to stronger existing partnerships and facilitating new collaborations (12). To further drive these efforts, the RRP has been engaging academia and industry in radiation biology/oncology at various workshops and symposia held at the annual meetings of societies, including the American Society of Radiation Oncology (ASTRO), American Association of Immunology, Society for Immunotherapy of Cancer, Radiosurgery Society and the Radiation Research Society (RRS) (e.g., Academic Industry Partnership in Radiation Oncology; Combination Therapy – Opportunities in Clinical Translation of Radiobiological Research; sessions in the 62nd Annual Meeting of the RRS, October 16–19, Kona, Hawaii). In their published opinion article, RRP members and leaders of the International Conference on Translational Research in Radio-Oncology and Physics for Health summarize recent radiation research workshops (13).
MEETING OBJECTIVES
Given the above ongoing efforts in radiation-drug combinations, the RRP organized a symposium to be held at the 63rd Annual Meeting of the RRS, Cancun, Mexico. Scholar-in-training, Kelly Falls (University of Iowa), and Pataje G. Prasanna (NCI) served as co-chairs and moderated the discussions. The objectives of this educational symposium were to: 1. outline the challenges in the development of next-generation radiation-drug combinations; 2. discuss approaches to improve scientific rationale and evidence to replace standard-of-care; and 3. consider ways to reduce normal tissue toxicity, with two broad overview talks followed by two talks as key illustrative examples, below:
Overviews
Challenges in developing new radiation-drug combinations - Richard Amos/Ricky Sharma, University College London, London, UK;
Changing the standard-of-care: The bumpy road from the bench to clinic - Yaacov Lawrence, Sheba Medical Center, Tel HaShomer, Israel.
Illustrative Examples
Antibody drug conjugate-targeted radiosensitization - Sunil Advani, University of California San Diego, La Jolla, CA.
Drug repurposing for tumor radiosensitization – Kelly Falls, University of Iowa, Iowa City, Iowa.
CHALLENGES IN DEVELOPING RADIATION-DRUG COMBINATIONS
The National Cancer Research Institute Clinical and Translational Radiotherapy Research Working Group (CTRad) formed a Joint Academia-Pharma Working Group with representatives from academia, industry, patient groups and regulatory bodies to accelerate the development of radiation-drug combinations (5). This Working Group collated evidence on the reluctance of the pharmaceutical industry to develop combinations of new drugs with radiotherapy (2, 3, 14). Although the reasons for this reluctance are complex, a fundamental lack of understanding of radiotherapy and its potential among scientists and clinicians within the pharmaceutical industry is a current issue. To address all of the current challenges, the Working Group’s consensus recommendations were to: 1. increase the number of novel drugs being successfully registered in combination with radiotherapy; 2. provide equal consideration to radiation-drug combinations to that of drug-drug combinations; 3. encourage the publication of regulatory guidance to the route to registration for novel drugradiotherapy combinations (Fig. 1); 4. consider trial designs that include clinically relevant early and intermediate end points of efficacy and toxicity; 5. ensure trial designs that take into account the potential changes to the standard-of-care during the recruitment and follow-up phase of a phase III clinical trial; 6. consider novel clinical trial methodologies; 7. ensure adequate radiotherapy quality assurance; 8. define the preclinical dataset and target population; and 9. involve patients and consumers from the earliest stages of trial concept and design and raise awareness of this area of clinical need among the general public (5).
FIG. 1.
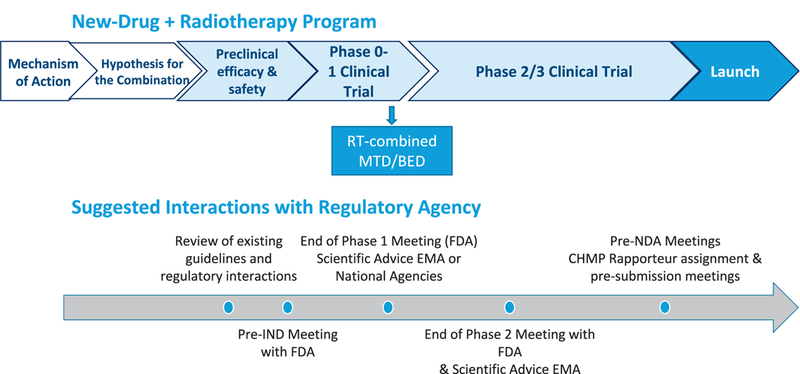
Proposed timelines for a new drug-radiotherapy combination, with suggested interactions with Regulatory Agency. MTD = maximum tolerated dose; BED = biologically effective dose; IND = investigational new drug; FDA = Food and Drug Administration; EMA = European Medicines Agency; NDA = new drug application; CHMP = Committee for Medicinal Products for Human Use. Modified and re-published with permission, from Sharma RA et al., “Clinical development of new drug-radiotherapy combinations.” Nat Rev Clin Oncol 2016; 13:627–42 (5).
The CTRad Working Group identified one particular barrier that was impairing progress in the field at multiple levels: the lack of infrastructure for developing the preclinical package for a new drug-radiotherapy combination. They cited as an exemplar of best practice the Radiotherapy-Drug Combinations Consortium (RaDCom), which was established by CTRad and the Cancer Research UK (CRUK) Centre for Drug Development (CDD) in 2013. RaDCom is a collaborative network of laboratories working in partnership with industry, CRUK and other funding bodies (15). It encourages industry to work with academic investigators to develop and deliver high-quality preclinical projects evaluating specific drug-radiotherapy combinations across a variety of clinical models at several collaborating laboratories. This ensures timely testing of the new combination to determine which cancer types and which molecular back-grounds the new combination has the greatest efficacy and the least toxicity to normal tissues. It is anticipated that this type of preclinical infrastructure will accelerate progress in providing the necessary evidence base for new combinations to advance to early-phase clinical trials.
CHANGING THE STANDARD-OF-CARE: A BUMPY ROAD FROM BENCH TO CLINIC
The manner in which the radiosensitizers, developed in the laboratory, move on to a clinical setting may appear deceptively simple. After a pathway of radiation resistance has been identified and a pharmacological agent discovered, which sensitizes cancer cells to ionizing radiation in vitro and in vivo, the next logical step is to move to the clinic to assess effects in humans and, if the balance between efficacy and toxicity are demonstrated in a sufficiently powered clinical trial, then establish a new “standard-of-care”. In reality, no drug has traversed this route. One example of an impediment to this process is hypoxia: while it is well-known mechanism of radiation resistance that has been targeted pharmacologically (e.g., tirapazamine, nitroimidazole) and by other interventions (e.g., hyperbaric oxygen, carbogen), there are no agents that are widely accepted to be clinically effective [the use of nimorazole in Denmark being the sole exception (16)]. Importantly, all radiosensitizers in clinical use (e.g., cisplatin, cetuximab, 5-FU, temozolomide), were developed as systemic monotherapy agents and their ability to radiosensitize was an afterthought. This talk outlined eight “challenges” that impede the path from the laboratory to the clinic for radiation-drug combinations.
Challenge No. 1: Questions to Answer Prior to First Human Trial
Once a new pharmaceutical entity has been discovered and validated in the preclinical setting, there are several steps that need to be completed before clinical testing is possible. These include careful documentation of pharmacokinetics, toxicity assessments and scaling up production both in terms of quantity and quality (clinical grade), a process governed by the Good Manufacturing Practices guidelines.
Challenge No. 2: Someone Else got There First
Drug development is not performed in a vacuum. Over the considerable time it takes to bring a new entity to development, it is very likely that clinical practice will have changed. For example, amifostine is a radioprotector demonstrated to decrease xerostomia, a significant toxicity from head-and-neck radiotherapy. By the time the phase III trial was published demonstrating the utility of Amifostine, intensity modulated radiotherapy techniques were introduced and to a large extent provided a physics solution to xerostomia, making amifostine largely irrelevant. In a phase III trial cetuximab added to radiotherapy improved overall-survival compared to radiotherapy alone for head-and-neck cancer, however, by the time of completion of the randomized phase III clinical study and its publication, standard-of-care for these cancers had shifted to combined modality cisplatin-radiotherapy treatment (17), thus, the “comparator” arm of radiation alone was outdated.
Challenge No. 3: Drug Development Costs a Lot of Money
The cost of drug development has risen rapidly over recent decades. It has been estimated that the research and development costs for a single new approved entity ranges between four and twelve billion dollars (18). Even though the clinical development of new drugs with radiation would likely add little to the overall costs, an indirect result of extreme drug development expenses is that pharmaceutical companies have adopted a “risk-averse” approach. Drug pipelines of different companies are surprisingly similar, and they tend to pursue well-trodden “paths to registration”, which do not include radiation-drug combinations.
Challenge No. 4: Many Clinical Trials Fail
Compared to other medical specialties, oncology drug development consistently appears at the bottom of tables assessing success in drug development. Only 40% of phase III trials in oncology are successful; the overall likelihood of a new entity being tested in a phase I trial achieving approval is a mere 5.1% (19).
Challenge No. 5: Clinical Trials may not Reflect the Real World
Clinical trials typically restrict participation to patients lacking co-morbidities. As a result, it is difficult to extrapolate benefits to the wider population of cancer patients who are older and less healthy. For example, an analysis of outcomes among pancreatic cancer patients receiving gemcitabine in the context of a clinical trial showed a steady improvement between the years 1995 and 2013 (20); a parallel analysis of population trends for pancreatic cancer outcomes in the U.S. demonstrated no improvement (21).
Challenge No. 6: Drug Companies are Not Interested in Radiation
Drug development is driven by the pharmaceutical industry, yet the pharmaceutical industry has its own priorities. A radiation-drug combination has not been the “path of registration” for any new drug, dampening industry enthusiasm. Our analysis of the database “ClinicalTrials.gov”(52) demonstrated that few cancer trials in either the phase I or phase III setting include radiation therapy, and that a very small proportion of these are solely funded by the pharmaceutical industry (Fig. 2). An additional reason for these findings is that very few physicians within the pharmaceutical industry have experience with radiation oncology. Thus, even when a radiation-drug combination concept arises there is a lack of expertise within the company to assess and develop it.
FIG. 2.
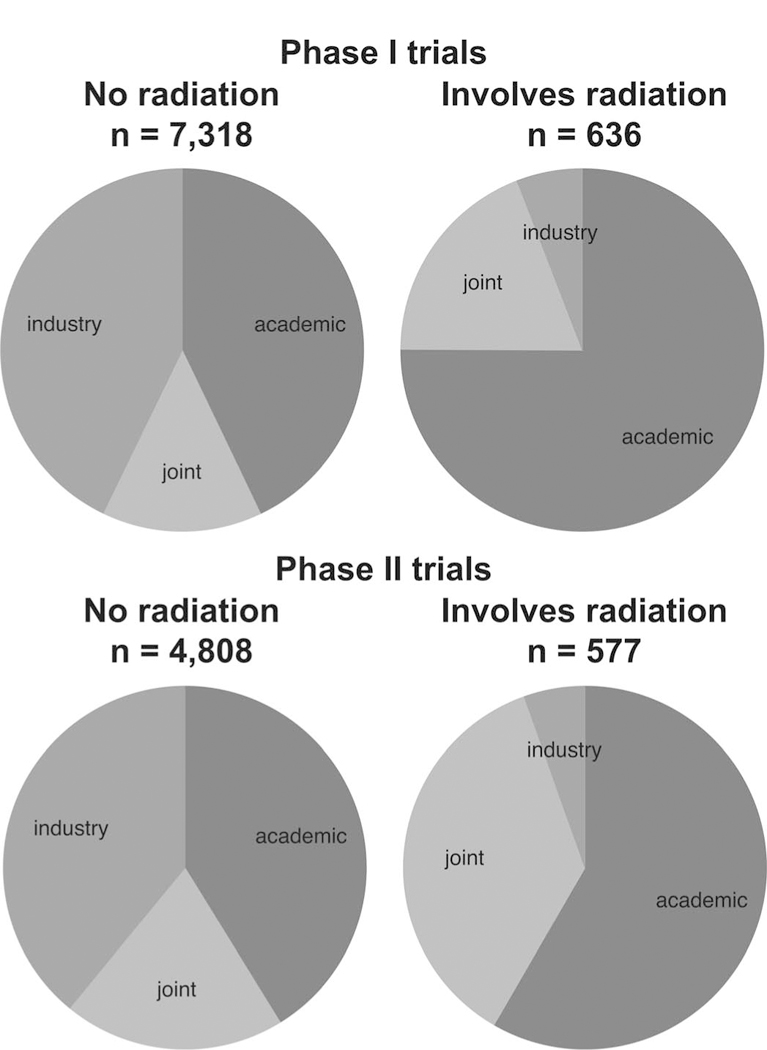
Funding of clinical trials, stratified by phase and whether or not the trial involves use of radiation therapy. Based on an analysis from database www.clinicaltrials.gov (52).
Challenge No. 7: Time is Our Enemy
Clinical drug development takes time, typically eight years from opening the first monotherapy clinical trial to publishing the results of a successful phase III trial (22). Clinical drug development with radiation typically starts six years after the opening of the first-in-human trial; consequently, there is simply not enough time for radiation-drug combinations to achieve commercial gain prior to patent expiry.
Challenge No. 8: Radiation Alone is Highly Effective
Pharmaceutical companies require an early readout of whether a particular radiation-drug combination is worth pursuing. The most familiar early readout is “response rate”, a measure of whether the tumor is growing or shrinking based on imaging. Since radiation alone almost always shrinks tumors initially, measuring “response rate” to assess a new radiation-drug combination is not useful. Consequently, alternative end points such as “metastases-free survival” or “pathological complete response rate” (if relevant) are needed to assess activity. Many of these end points are less familiar and occur late, delaying when companies can make a go/no-go decision, making radiation-drug combination projects less attractive from the outset.
EXAMPLE 1: ANTIBODY DRUG CONJUGATES AS A NEW FORM OF RADIOSENSITIZERS
To improve the therapeutic index of radiotherapy, drugs that sensitize tumor cells to radiation are used. As mentioned above, the current clinically effective radiosensitizers are almost exclusively nontargeted cytotoxic chemotherapies. While cytotoxic chemotherapies increase tumor control when combined with ionizing radiation, such drugs unfortunately may cause increased normal tissue damage in the irradiated field as well as systemic toxicities. While the combination of cetuximab with radiotherapy has been shown to be beneficial over radiotherapy alone, the addition of cetuximab to cytotoxic chemo-radiotherapy has failed to improve survival in two phase III clinical trials, the first involving head and neck cancer patients and the second in patients with non-small cell lung cancer (NSCLC) (23, 24). Theoretically, more potent radiosensitizers should increase tumor kill and improve patient outcomes. However, the clinical utility of increasingly potent radiosensitizers is curtailed by both systemic side effects and potential toxicity to normal tissue surrounding the irradiated tumor target. While innovative radiation delivery techniques can reduce the amount of normal tissue receiving a high dose, this may be at the expense of more normal tissue being exposed to “some dose”. An alternative strategy is to use more potent radiosensitizers whose delivery is targeted to tumors.
A readily apparent solution appears to be antibody drug conjugates (ADC). ADC technology splits the roles of tumor targeting and cell killing into two molecules (25). Accordingly, tumor targeting is achieved by antibodies that recognize cell surface receptors expressed preferentially on tumor cells. Tumor kill is mediated not by receptor signal inhibition of the antibody but rather by the attached drug payload, i.e., “warhead”. After cell surface receptor binding and internalization, the bound warhead drug is released through the actions of endosomal proteases. Due to the inherent specificity of this approach, increasingly potent radiosensitizers can be attached to antibodies. ADC represent a novel strategy to deliver more potent radiosensitizing drugs in a biomarker-driven fashion (Fig. 3).
FIG. 3.
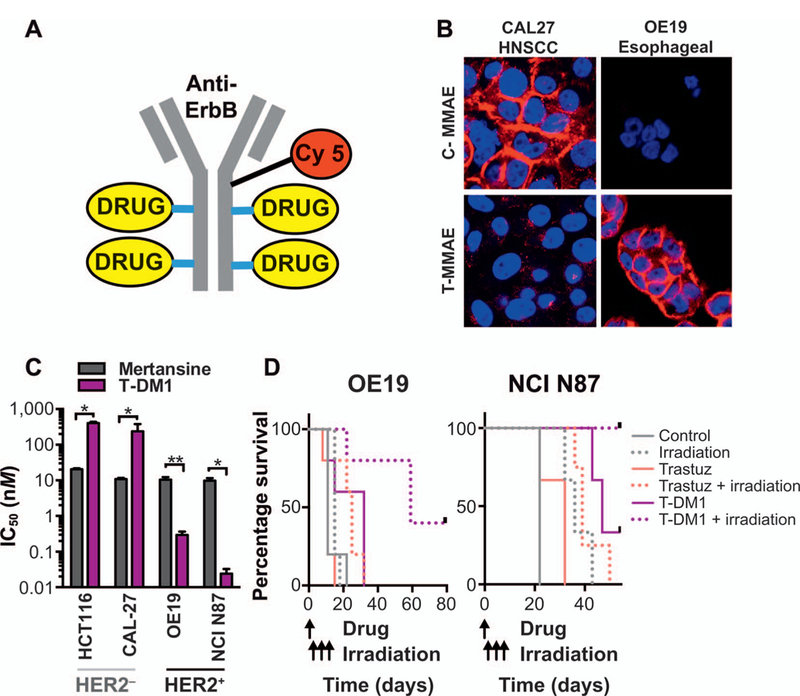
Antibody drug conjugate targeted radiosensitization. Panel A: Cy5-labeled ADC with four molecules of radiosensitizing drug conjugated. Panel B: Cy5-labeled cetuximab (C-MMAE) or trastuzumab (T-MMAE) ADC bound specifically to EGFR+ cells (CAL27) and HER2+ cells (OE19), respectively. Panel C: Conjugating radiosensitizing maytansinoid to trastuzumab (T-DM1) restricts maytansinoid toxicity to HER2-expressing tumor cells. Unconjugated maytansinoid (mertansine) is equally cytotoxic to tumor cells irrespective of HER2 status. Panel D: T-DM1 in combination with irradiation results in significantly enhanced survival in preclinical murine tumor models. Survival of mice bearing HER2-expressing OE19 or NCI-N87 tumors. Trastuzumab or T-DM1 were given intravenously followed by localized irradiation to the tumor. Modified and re-published with permission, from Adams et al. “Anti-tubulin drugs conjugated to anti-ErbB antibodies selectively radiosensitize.” Nat Commun 2016; 7:13019 (26).
As stated above, the cost and regulatory burden associated with developing new oncologic drugs is a formidable obstacle in testing new radiation-drug combinations. An advantage of testing ADC with radiotherapy is that ADC have already begun to be evaluated clinically as monotherapies. Safety and efficacy have been established for two ADC. Brentuximab vedotin (ADCTERIS®, Seattle Genetics®, Inc.) and T-DM1 (Kadcyla®, Roche-Genentech) have demonstrated benefit in patients with CD30-expressing lymphomas and HER2-expressing metastatic breast cancer, respectively. Brentuximab vedotin is composed of a CD30-recognizing antibody conjugated to monomethyl auristatin E (MMAE). For T-DM1, the HER2-recognizing trastuzumab is conjugated to emtansine (maytansinoid) (Fig. 3). Mechanistically, the ADC warheads MMAE and maytansinoids are potent anti-tubulins that radiosensitize in cell culture (26). In gold standard clonogenic survival assays for radiosensitization, MMAE or maytansinoid increased cell-killing when combined with radiation. Importantly, whereas MMAE and maytansinoid are indiscriminate radiosensitizers to all tumor cells (and likely any cycling normal cell), conjugation to ErbB antibodies restricted radiosensitization to target positive ErbB-expressing, but not ErbB-negative, tumors.
Of direct translational relevance for ADC-mediated radiosensitization, HER2 overexpression has been reported in a proportion of solid tumors from many histologies including NSCLC, esophageal, gastric and bladder cancer (26). Importantly, patients with such cancers are treated with concurrent cytotoxic chemotherapy and radiotherapy resulting in significant morbidity and outcomes that are less than optimal. Given that T-DM1 is clinically approved in metastatic HER2-expressing breast cancer, a path toward clinical testing of T-DM1 in combination with radiotherapy may provide an opportunity to cost-effectively assess the paradigm of using ADC in combination with radiotherapy. As noted above, challenges are present that hinder development of ADC-radiation combinations, including pharmaceutical interest in radiation, the time spent on ADC’s initial testing as a monotherapy and the limited patient population that may express a targeted bio-marker (e.g., HER2 for T-DM1).
EXAMPLE 2: DRUG REPURPOSING FOR TUMOR SENSITIZATION
Given the expense and minimal success of developing new drugs for cancer therapy, repurposing of compounds already in use is a strategy for overcoming the constraints. Through functional screening, a number of old therapeutics have emerged as anti-cancer agents, often based on an understanding of their mechanisms of action from the perspective of modern scientific techniques and knowledge. Some of these agents have been shown to improve therapeutic outcomes when combined with standard chemotherapies and radiation. A recently published prospective observational cohort study of stage II or III rectal cancer patients showed that the use of aspirin during chemoradiation therapy led to higher rates of tumor downstaging and improved five-year progression-free survival (27). Another published study of locally advanced rectal cancer showed that the diabetes drug metformin in combination with radiation and chemotherapy led to higher rates of complete response, disease-free and overall survival (28). Given this potential efficacy, both of these drugs are being investigated for treatment of numerous cancer types. According to clinicaltrials.gov database, there are 65 active studies and 131 total studies being conducted with aspirin in cancer patients as well as 132 active studies and 307 total studies being conducted with metformin in cancer patients. The vast number of studies with just these two agents highlights the potential for repurposing old drugs for cancer therapy.
To streamline the approach of discovering drugs for oncologic repurposing, tumor biology and radiation biology must be considered. Our work at the University of Iowa on drug repurposing has focused on compounds that lead to oxidative stress in cancer cells (Fig. 4). Cancer cells have increased levels of reactive oxygen species (ROS) such as superoxide (O2·−) and hydrogen peroxide (H2O2), leading to increased susceptibility to oxidative stress as well as radiation and chemotherapy (29). This translates into greater sensitivity to radiation-drug therapies that involve oxidative mechanisms. It has been shown that combined inhibition of the glutathione-dependent and thioredoxin-dependent H2O2-scavenging pathways with the repurposed drug auranofin in combination with buthionine sulfoxamine (BSO) increased cancer cell-killing alone and in combination with radiation and chemotherapy (30, 31). Auranofin, which has been used in the treatment of rheumatoid arthritis, is an inhibitor of thioredoxin reductase (32). BSO depletes glutathione (GSH) by inhibiting γ-glutamylcysteine synthetase (33). Other published studies have also shown that overproduction of H2O2 in cancer cells using high-dose vitamin C can cause cancer cell death alone and in combination with chemotherapy and radiation (34–36). Notably, these studies with vitamin C have shown safety and potential efficacy in both preclinical and clinical studies of glioblastoma and NSCLC (34).
FIG. 4.

Repurposed drugs cause oxidative stress in cancer cells. BSO and AUR disrupt glutathione and thioredoxin-dependent peroxide scavenging pathways. DSF and DPEN in combination with copper as well as vitamin C in combination with iron induce production of superoxide and peroxide. BSO = buthionine sulfoxamine; AUR = auranofin; DSF = disulfiram; DPEN = d-penicillamine; GSH = glutathione; Trx = thioredoxin; TR = thioredoxin reductase; PRx = peroxiredoxin; GPx = glutathione peroxidase; H2O2 = hydrogen peroxide; O2·− = superoxide.
To further exploit the differences between tumor and normal cells, we have also focused on the differences in metal ions between these cell populations. Studies have shown that the differential toxicity of vitamin C on tumor and normal cells is due to varying levels of labile iron in these cells (34). The presence of iron causes increased oxidative stress in cancer cells, leading to increased toxicity. Interestingly, cancer tissue has elevated copper levels when compared with normal tissue (37, 38). This elevated copper can interact with copper chelators such as D-penicillamine and disulfiram, causing induction of oxidative stress (39– 41). D-penicillamine is a repurposed drug that was previously used for treatment of Wilson’s disease and rheumatoid arthritis (41). Disulfiram is used for the treatment of alcoholism (42). Both of these agents are now being investigated as anti-cancer drugs and have shown promise when combined with both chemotherapy and radiation (39, 43, 44). Notably, studies have shown that disulfiram is safe and tolerable in patients treated for both glioblastoma and NSCLC in combination with standard chemotherapies (43, 44). However, further studies are necessary to prove its efficacy and safety in combination with radiation. Nonetheless, the mechanism of action involves induction of oxidative stress, which will likely be synergistic with radiation therapy, especially considering the success of other oxidative therapies. This potential for synergy highlights the advantage of redox biology as a pharmacologic target for cancer therapy. A large number of old drugs are inducers of oxidative stress and may be readily repurposed as cancer therapeutics. Additionally, these drugs have been shown to be safe and tolerable and may be easily combined with radiation in a cost-effective manner. This is an advantage over new drug development and may lead to novel radiation-drug combinations that improve patient outcomes. A clear disadvantage to repurposing is that funding from company sources is nearly nonexistent, and most funding comes from public entities. Thus, while repurposing can provide a faster, more affordable method of radiation-drug development, there are limited resources to make this happen.
SUMMARY AND CONCLUSIONS
Many attendees participated in lively discussions, and we recognize that all the discussions might not have been captured. However, major discussions are highlighted below.
While the focus of radiation-drug combinations is to improve the efficacy of tumor cell-killing, it is important to minimize normal tissue toxicities, because the therapeutic gain is dependent on the difference between efficacy and toxicity. A general nonprescriptive drug development process for radiation-effect modulators has been described elsewhere (12, 45). In general, regulatory agencies are concerned with safety and demonstration of clinical benefit. Since animal models provide an opportunity to address safety and efficacy under controlled conditions, preclinical studies are essential. However, one impediment to drug development has been the irreproducibility of preclinical data (46); thus, there is an urgent need to improve reproducibility and translatability of preclinical data to fully exploit opportunities for molecularly-targeted therapeutics involving radiation and radiochemotherapy (10). While the clonogenic assay remains as the state-of-the-art in vitro assay for initial screening, in vivo studies are essential to confirm in vitro findings, and to define mechanisms of action and treatment-induced modification of the tumor microenvironment. Selection of appropriate end points is important for in vivo studies. Testing radiation-effect modulators as a part of clinical standard-of-care, which includes radiochemotherapy in most cases, is essential (10). Several known and recently identified targets can prevent or mitigate normal tissue injury. Basic and translational research and focused clinical trials are needed to identify optimal agents and strategies for therapeutic use (47).
With respect to radiosensitizers, the hitherto most successful formula for evaluation appears to involve choosing a drug, which is demonstrated to be active on its own against a given type of cancer in the advanced setting, and adding it before, during or after radiation therapy in the locally advanced setting. For example, fluorouracil, cisplatin, gemcitabine and, most recently, durvalumab, have reinforced the success of this strategy. The NCI’s RRP and Chemotherapy Evaluation Program has in the past decade tried a more “scientific” approach, i.e., attempting to combine the drug with radiation based on its putative mechanism of action, which seeks to exploit a vulnerability in the cancer and/or interaction with key targets such as DNA damage repair pathways. Some examples include inhibitors of PARP, MDM2, ribonucleotide reductase, IAP, HSP90 and chromosome region maintenance 1 (CRM1 shows high expression in certain type of cancers). However, to date there have been no spectacular successes for this strategy, perhaps due to the complexity of the problem and dearth of well-designed studies. In radiation oncology research, radiation is now considered a “drug” in which the type, dose and fractionation can be varied to produce the desired effect; therefore, it has become important that radiation be considered as an essential participant in precision medicine (9).
Targeted therapy will be ineffective in the absence of the target and clonal selection will dominate tumor recurrence. However, tumors that recurred locally after patients had received radiation or chemoradiation have not been systematically studied to identify new targets as tumors recur and develop. Thus, there is little information on the mechanisms of development of resistance to therapies and availability of new targets during the course of tumor development or recurrence. Novel agents, in most cases, are evaluated with cell lines or xenografts derived from naїve biopsies, or mouse models, in which experimental therapy is provided at diagnosis but not after standard therapy. Thus, since recurrent tumors are likely to be genetically divergent from pre-therapy disease, and current experimental models and approaches fail to model a recurrent tumor, genetic divergence with the loss of a target at recurrence could account for the lack of success seen in clinical trials with radiation-drug combinations (48). A novel murine Trp53 and Keap1 deletion model of lung squamous cell carcino-mas (LSSC) has been recently developed (49). Trp53 and Keap1 mutations are frequent in human LSSC. Deletion of KEAP1 promotes tumor aggressiveness, metastasis and resistance to oxidative stress and radiotherapy. KEAP1/NRF2 mutation status could predict the risk of local recurrence after radiotherapy in patients with NSCLC and could be detected in circulating tumor DNA. Thus, KEAP1/NRF2 mutations could serve as predictive biomarkers and offer opportunities to personalization of therapeutic strategies for NSCLCs (49).
Cancer drug development paradigms designed for “drug-alone” approaches may be inappropriate for combined radiotherapy strategies. Some of the challenges outlined here have already been addressed by the NCI-RTOG (2), including the use of new clinical trial designs, such as a risk-stratified model for drug dose escalation, time-to-event continual reassessment, randomized phase II “screening” trials, and the use of surrogate end points, such as pathological response (2). Lack of commercial funding for radiation studies is a major barrier in the highly regulated environment of drug development.
The following issues were also discussed at the symposium: 1. the need to prioritize radiation-drug early; 2. the need to address lack of enthusiasm from industry in the development of radiation-drug combinations, and to encourage academic-small business partnerships to fill the critical gaps in funding for the development of radiation-drug combinations (12); 3. the use of a sound scientific basis, considering radiobiology for both mechanisms of cell death and route to registration; 4. consideration of effects on tumor versus normal cells to maximize therapeutic effects and minimize normal tissue damage; 5. consideration of the mechanisms of radiation failure, maximizing initial therapeutic response and targeting of radiation-resistant cells downstream (cancer stem cells, quiescent cells, hypoxic cells); 6. inclusion of biomarkers; and 7. consideration of the level of confidence necessary in the generated scientific evidence to conduct first-in-human clinical trials, given the irreproducibility of preclinical data (46) and the need for better preclinical models (10).
As we progress toward developing novel standard-of-care treatment approaches, replacing the current ones with radiation with the goal of achieving complete remission, it is clear that the use of agents that act via a complementary mechanism of action to the current treatment regimen is essential, so that efficacy of such combinations is augmented, while toxicity is mitigated. These drug combination approaches could be based on the following.
Rational Sequencing of Radiation-Drug Combinations
It is imperative to identify the right target, during tumor progression or recurrence, and the treatment-induced inflection point for a given target, by understanding the mechanism of action for each radiation dose alone or with chemotherapy. This kind of rational sequencing at the right window of opportunity to enhance efficacy will maximize sensitization.
Exploitation of Radiation-Induced Immune Modulation
Traditionally, radiation has been used as an agent to induce DNA damage, and thereby cell killing. In a changing paradigm of the cancer biology landscape, definitive evidence is accumulating, which demonstrates that radiation serves to mimic viral infection leading to host immune cascade. The immune modulatory potential of radiation therapy needs to be carefully exploited by developing agents that can augment these effects leading to a full-blown host immune attack on every single tumor cell in both primary and metastatic lesions (50). Similarly, standard chemoradiotherapy is known to cause leukopenia and immune suppression (51). Therefore, it will be important to take this aspect into consideration when developing radiation-drug combinations.
Radiotherapy-Specific Drug Development
The above two forward-thinking concepts can be adapted in new drug discovery settings specifically for use with radiation therapy that can include the development of new ADCs or repurposed drugs.
This symposium was a success. Key issues in the development and translation of radiation-drug combinations were highlighted and addressed, and participants comprised a cross section of clinicians, scientists and scholars-in-training. In key illustrative example talks, several strategies were addressed for the development and translation to the clinic of novel or repurposed radiation-drug combinations, in light of the suggested guidelines. In broad overview talks, challenges were identified. As researchers continue in their endeavors to find new treatment options for patients, forums like this symposium provide ample opportunities to discuss relevant issues for all those involved. These symposia also provide an efficient and effective means of networking, where participants can benefit from broad and didactic interactions with other researchers. In addition, they provide opportunities to educate and train the next generation of scientists in this emerging area in radiation biology/oncology research.
ACKNOWLEDGMENTS
We thank all the participants for engaged discussions. The symposium was primarily supported by the RRS and the NCI’s RRP. Additional support was provided by the NIHR University College London Hospitals Biomedical Research Centre, the UCL Experimental Cancer Medicines Centre and Cancer Research, UK (to RAS), the NIH/NCI (grant nos. R01CA215081A1, R03CA219744 and R21CA205765 to SJA) and the Rosetrees Trust, UK (to YL). The views expressed in this article are those of authors. No endorsement by the NCI, NIH, HHS or any other U.S. Government Agencies has been given, implied or inferred. YL has received research funding from Karyopharm Therapeutics, Checkmate Pharmaceuticals and Bristol-Myers Squibb. RAS is a consultant for Affidea, Astra Zeneca, Bayer, Boston Scientific, BTG, Cancer Research UK, Cancer Research Technology, Eisai, Ipsen, Sirtex, Terumo and Varian, and has received research funding from Cancer Research UK, Sirtex and BTG. SJA is a consultant for Astellas and Coastar Therapeutics and is co-inventor on U.S. patent applications filed by UCSD on radiosensitizers.
Footnotes
NRG Oncology is a National Clinical Trials Network (NCTN) group created through the coordinated efforts of the National Surgical Adjuvant Breast and Bowel Project (NSABP), the Radiation Therapy Oncology Group (RTOG), and the Gynecologic Oncology Group (GOG). NRG is not an acronym; it was created to represent the new group NSABP/RTOG/GOG.
REFERENCES
- 1.Baumann M, Krause M, Overgaard J, Debus J, Bentzen SM, Daartz J, et al. Radiation oncology in the era of precision medicine. Nat Rev Cancer 2016; 16:234–49. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence YR, Vikram B, Dignam JJ, Chakravarti A, Machtay M, Freidlin B, et al. NCI-RTOG translational program strategic guidelines for the early-stage development of radiosensitizers. J Natl Cancer Inst 2013; 105:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ataman OU, Sambrook SJ, Wilks C, Lloyd A, Taylor AE, Wedge SR. The clinical development of molecularly targeted agents in combination with radiation therapy: a pharmaceutical perspective. Int J Radiat Oncol Biol Phys 2012; 84:e447–54. [DOI] [PubMed] [Google Scholar]
- 4.Ringborg U, Bergqvist D, Brorsson B, Cavallin-Stahl E, Ceberg J, Einhorn N, et al. The Swedish Council on Technology Assessment in Health Care (SBU) systematic overview of radiotherapy for cancer including a prospective survey of radiotherapy practice in Sweden 2001–summary and conclusions. Acta Oncol 2003; 42:357–65. [DOI] [PubMed] [Google Scholar]
- 5.Sharma RA, Plummer R, Stock JK, Greenhalgh TA, Ataman O, Kelly S, et al. Clinical development of new drug-radiotherapy combinations. Nat Rev Clin Oncol 2016; 13:627–42. [DOI] [PubMed] [Google Scholar]
- 6.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006; 354:567–78. [DOI] [PubMed] [Google Scholar]
- 7.Bonner JA, Raisch KP, Trummell HQ, Robert F, Meredith RF, Spencer SA, et al. Enhanced apoptosis with combination C225/radiation treatment serves as the impetus for clinical investigation in head and neck cancers. J Clin Oncol 2000; 18:47s–53s. [PubMed] [Google Scholar]
- 8.Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009; 373:1525–31. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed MM, Coleman CN, Mendonca M, Bentzen S, Vikram B, Seltzer SM, et al. Workshop Report for cancer research: Defining the shades of Gy: Utilizing the biological consequences of radiotherapy in the development of new treatment approaches-meeting viewpoint. Cancer Res 2018; 78:2166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman CN, Higgins GS, Brown JM, Baumann M, Kirsch DG, Willers H, et al. Improving the predictive value of preclinical studies in support of radiotherapy clinical trials. Clin Cancer Res 2016; 22:3138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone HB, Bernhard EJ, Coleman CN, Deye J, Capala J, Mitchell JB, et al. Preclinical data on efficacy of 10 drug-radiation combinations: evaluations, concerns, and recommendations. Transl Oncol 2016; 9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasanna PG, Narayanan D, Hallett K, Bernhard EJ, Ahmed MM, Evans G, et al. Radioprotectors and radiomitigators for improving radiation therapy: the Small Business Innovation Research (SBIR) gateway for accelerating clinical translation. Radiat Res 2015; 184:235–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coleman CN, Prasanna PGS, Bernhard EJ, Buchsbaum JC, Ahmed MM, Capala J, et al. Accurate, precision radiation medicine: A meta-strategy for impacting cancer care, global health, nuclear policy, and mitigating radiation injury from necessary medical use, space exploration and potential terrorism. Int J Radiat Oncol Biol Phys 2018; 101:250–3. [DOI] [PubMed] [Google Scholar]
- 14.Harrington KJ, Billingham LJ, Brunner TB, Burnet NG, Chan CS, Hoskin P, et al. Guidelines for preclinical and early phase clinical assessment of novel radiosensitisers. Br J Cancer 2011; 105:628–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CTRad. NCRI Radiotherapy-Drug Combinations Consortium (RaDCom) London: National Cancer Research Institute Team (NCRIT), CTRad; 2015. (https://bit.ly/2EYZUW0). [Google Scholar]
- 16.Metwally MA, Frederiksen KD, Overgaard J. Compliance and toxicity of the hypoxic radiosensitizer nimorazole in the treatment of patients with head and neck squamous cell carcinoma (HNSCC). Acta Oncol 2014; 53:654–61. [DOI] [PubMed] [Google Scholar]
- 17.ASTRO. New ASTRO guideline establishes standard of care for curative treatment of oropharyngeal cancer with radiation therapy Arlington, VA: American Society for Radiation Oncology; 2018. (https://bit.ly/2lwnDBx) [Google Scholar]
- 18.Wikipedia. Cost of drug development San Francisco: Wikipedia; 2018. (https://bit.ly/2lC6Wot) [Google Scholar]
- 19.Thomas DW, Burns J, Audette J, Carrroll A, Dow-Hygelund C, Hay M. Clinical development success rates 2006–2015 Washington, DC: BioMedTracker, Amplion and Biotechnology Innovation Organization (BIO); 2016. (https://bit.ly/1UfCn1S) [Google Scholar]
- 20.Rahib L, Fleshman JM, Matrisian LM, Berlin JD. Evaluation of pancreatic cancer clinical trials and benchmarks for clinically meaningful future trials: a systematic review. JAMA Oncol 2016; 2:1209–16. [DOI] [PubMed] [Google Scholar]
- 21.Golan T, Sella T, Margalit O, Amit U, Halpern N, Aderka D, et al. Short- and long-term survival in metastatic pancreatic adenocarcinoma, 1993–2013. J Natl Compr Canc Netw 2017; 15:1022–7. [DOI] [PubMed] [Google Scholar]
- 22.Blumenfeld P, Pfeffer RM, Symon Z, Den RB, Dicker AP, Raben D, et al. The lag time in initiating clinical testing of new drugs in combination with radiation therapy, a significant barrier to progress? Br J Cancer 2014; 111:1305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 2014; 32:2940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015; 16:187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullard A Maturing antibody-drug conjugate pipeline hits 30. Nat Rev Drug Discov 2013; 12:329–32. [DOI] [PubMed] [Google Scholar]
- 26.Adams SR, Yang HC, Savariar EN, Aguilera J, Crisp JL, Jones KA, et al. Anti-tubulin drugs conjugated to anti-ErbB antibodies selectively radiosensitize. Nat Commun 2016; 7:13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Restivo A, Cocco IMF, Casula G, Scintu F, Cabras F, Scartozzi M, et al. Aspirin as a neoadjuvant agent during preoperative chemoradiation for rectal cancer. Br J Cancer 2015; 113:1133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skinner HD, Crane CH, Garrett CR, Eng C, Chang GJ, Skibber JM, et al. Metformin use and improved response to therapy in rectal cancer. Cancer Med 2013; 2:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR. Increased levels of superoxide and hydrogen peroxide mediate the differential susceptibility of cancer cells vs. Normal cells to glucose deprivation. Biochem J 2009; 418:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodman SN, Spence JM, Ronnfeldt TJ, Zhu Y, Solst SR, O’Neill RA, et al. Enhancement of radiation response in breast cancer stem cells by inhibition of thioredoxin-and glutathione-dependent metabolism. Radiat Res 2016; 186:385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fath MA, Ahmad IM, Smith CJ, Spence J, Spitz DR. Enhancement of carboplatin-mediated lung cancer cell killing by simultaneous disruption of glutathione and thioredoxin metabolism. Clin Cancer Res 2011; 17:6206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papaioannou M, Mylonas I, Kast RE, Bruning A. Disulfiram/copper causes redox-related proteotoxicity and concomitant heat shock response in ovarian cancer cells that is augmented by auranofin-mediated thioredoxin inhibition. Oncoscience 2014; 1:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009; 458:780–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Wagner BA, Cramer-Morales KL, Furqan M, et al. O2- and H2O2-mediated disruption of fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell 2017; 31:487–500.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du J, Cieslak JA 3rd, Welsh JL, Sibenaller ZA, Allen BG, Wagner BA, et al. Pharmacological ascorbate radiosensitizes pancreatic cancer. Cancer Res 2015; 75:3314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Bradley MD, Wagner BA, Buettner GR, et al. Redox active metals and H2O2 mediate the increased efficacy of pharmacological ascorbate in combination with gemcitabine or radiation in pre-clinical sarcoma models. Redox Biol 2018; 14:417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zowczak M, Iskra M, Torlinski L, Cofta S. Analysis of serum copper and zinc concentrations in cancer patients. Biol Trace Elem Res 2001; 82:1–8. [DOI] [PubMed] [Google Scholar]
- 38.Gupte A, Mumper RJ. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev 2009; 35:32–46. [DOI] [PubMed] [Google Scholar]
- 39.Sciegienka SJ, Solst SR, Falls KC, Schoenfeld JD, Klinger AR, Ross NL, et al. D-penicillamine combined with inhibitors of hydroperoxide metabolism enhances lung and breast cancer cell responses to radiation and carboplatin via H2O2-mediated oxidative stress. Free Radic Biol Med 2017; 108:354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allensworth JL, Evans MK, Bertucci F, Aldrich AJ, Festa RA, Finetti P, et al. Disulfiram (DSF) acts as a copper ionophore to induce copper-dependent oxidative stress and mediate anti-tumor efficacy in inflammatory breast cancer. Mol Oncol 2015; 9:1155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupte A, Mumper RJ. An investigation into copper catalyzed D-penicillamine oxidation and subsequent hydrogen peroxide generation. J Inorg Biochem 2007; 101:594–602. [DOI] [PubMed] [Google Scholar]
- 42.Skrott Z, Mistrik M, Andersen KK, Friis S, Majera D, Gursky J, et al. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature 2017; 552:194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nechushtan H, Hamamreh Y, Nidal S, Gotfried M, Baron A, Shalev YI, et al. A phase IIb trial assessing the addition of disulfiram to chemotherapy for the treatment of metastatic non-small cell lung cancer. Oncologist 2015; 20:366–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J, Campian JL, Gujar AD, Tran DD, Lockhart AC, DeWees TA, et al. A phase I study to repurpose disulfiram in combination with temozolomide to treat newly diagnosed glioblastoma after chemoradiotherapy. J Neurooncol 2016; 128:259–66. [DOI] [PubMed] [Google Scholar]
- 45.Prasanna PG, Stone HB, Wong RS, Capala J, Bernhard EJ, Vikram B, et al. Normal tissue protection for improving radiotherapy: Where are the gaps? Transl Cancer Res 2012; 1:35–48. [PMC free article] [PubMed] [Google Scholar]
- 46.Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature 2012; 483:531–3. [DOI] [PubMed] [Google Scholar]
- 47.Citrin DE, Prasanna PGS, Walker AJ, Freeman ML, Eke I, Barcellos-Hoff MH, et al. Radiation-induced fibrosis: mechanisms and opportunities to mitigate. Report of an NCI Workshop, September 19, 2016. Radiat Res 2017; 188:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrissy AS, Garzia L, Shih DJ, Zuyderduyn S, Huang X, Skowron P, et al. Divergent clonal selection dominates medullo-blastoma at recurrence. Nature 2016; 529:351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeong Y, Hoang NT, Lovejoy A, Stehr H, Newman AM, Gentles AJ, et al. Role of KEAP1/NRF2 and TP53 mutations in lung squamous cell carcinoma development and radiation resistance. Cancer Discov 2017; 7:86–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmed MM, Hodge JW, Guha C, Bernhard EJ, Vikram B, Coleman CN. Harnessing the potential of radiation-induced immune modulation for cancer therapy. Cancer Immunol Res 2013; 1:280–4. [DOI] [PubMed] [Google Scholar]
- 51.Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol 2018; 123:42–51. [DOI] [PubMed] [Google Scholar]
- 52.NIH, U.S. National Library of Medicine ClinicalTrials.gov Bethesda, MD: National Institutes of Health; (http://clinicaltrials.gov/) [Google Scholar]


