Abstract
Fludioxonil is a phenylpyrrole pesticide that is applied to fruit and vegetable crops post-harvest to minimize losses to mold, both during transport and at point of sale. Its effectiveness is reflected in the dramatic increase in its production/usage since its introduction in 1994, an increase that has peaked in recent years as it became licenced for use abroad. Recently, doubts as to the nature of its mechanism of action have been raised. Given that the pesticide has long been known to induce stress intermediates in target and non-target organisms alike, the lack of a firmly established mechanism might be cause for concern. Troubling reports further delineate a capacity to disrupt hepatic, endocrine and neurological systems, indicating that fludioxonil may represent a health threat to consumers. In the absence of a clear, safe mechanism of action, fludioxonil should be re-evaluated for its potential to impact human health.
Keywords: Phenylpyrrole, Pyrrolnitrin, pesticide, toxicity, oxidative, aldehydic
Introduction
Fungal diseases of fruit-bearing plants and vegetables are a serious concern for those who grow, transport and sell these commodities. Fungal infection leads to losses in the yield, quality and potential shelf-life of virtually every agricultural product, and modern monoculture techniques tend to exacerbate the vulnerability of crops to the spread of such pathogens. Beyond simply impacting the appearance, taste and nutritional value of harvested products, infection with many common fungal pathogens can contaminate crops with hazardous mycotoxins, rendering them inedible and unsalable. In order to combat these issues, modern agrochemical concerns have sought to develop fungicides that maximize efficacy yet expose humans, animals and the crops themselves to a minimal toxicological impact.
Fludioxonil is one of two existing commercial phenylpyrrole fungicides (the other being fenpiclonil) derived from the antibiotic pyrrolnitrin (Arima et al., 1964; Kilani and Fillinger, 2016; Gehmann et al., 1990). Fludioxonil is currently cleared for application to over 900 agricultural products and is included, often as the primary ingredient, in over 30 different pesticide formulations produced by Syngenta, Bayer and (formerly) Monsanto (Code of Federal Regulations for Fludioxonil, 2005). While originally licensed for use in the preservation of seed stocks, the use of this fungicide has expanded substantially in recent years to include a variety of agricultural and domestic treatments, including post-harvest applications. In 2014, sales of fludioxonil exceeded 250 million US dollars, establishing fludioxonil as a major force, and numerous international markets have chosen to permit fludioxonil use since then. As Kilani and Fillinger published an in depth review of the phenylpyrroles in 2016 (Kilani and Fillinger, 2016), focusing upon the success of these fungicides and the fact that crop pathogens have been surprisingly slow to develop immunity in the field, we are not inclined to dispute or rehash this review. Instead we will focus upon what is known regarding the phenylpyrrole mechanism of action and observations that may tend to call into question the conclusion that they are toxic to fungi alone.
Fludioxonil has long been purported to act by inhibiting class lll hybrid histidine kinases (HHK) that are peculiar to fUngi. These HHKs act to regulate the HOG osmolarity response pathway (Yoshimi et al., 2005), and were believed to respond to fludioxonil by triggering an overproduction of glycerol that caused cells to burst (Lew, 2010). This model went unquestioned for many years because rare instances of fludioxonil resistance, usually induced artificially under laboratory conditions, were most frequently derived from mutations in the Hog1 pathway(Ochiai et al., 2001). Further, the class lll HHKs were requisite for sensitivity (Kojima et al., 2004; Yoshimi et al., 2005), while constitutively active forms of these HHKs conferred fluidioxonil resistance (Furukawa et al., 2012). These HHKs were not found in plants, animals or humans, and were touted for two decades as the putative target of fludioxonil, since recombinant expression of the same HHK gene in previously resistant species, like Saccharomyces cerevisea, engenders fludioxonil sensitivity (Motoyama, Ohira, et al., 2005; Motoyama, Kadokura, et al., 2005). Were this indeed true, fludioxonil would seem an optimal choice for post-harvest applications, but the details of this mechanism remained elusive.
It is notable that several other classes of anti-fungals, including the dicarboximides, polyketides and the aromatic antifungals, show evidence of working along similar lines, signaling through the HOG pathway (Vetcher et al., 2007; Yoshimi et al., 2005). It is possible that all of their mechanisms of toxicity are derived from some common root (Table 1). While action through the HOG pathway is not in question, Lawry et al furnished evidence against direct action upon the HHK as the operative mechanism of fludioxonil (Lawry et al., 2017). As of this writing, promotional materials for fludioxonil within the pesticide industry exclusively mention an alternate model in which fludioxonil acts by inhibiting transport-associated phosphorylation of glucose, derived from work published in 1995 (Jespers and Dewaard, 1995) (though only fenpiclonil was investigated). Such a mode of action would pose as little risk to non-target organisms as the previously proposed mechanism, but evaluating the validity of this new claim requires the perspective of a complete record of research into the phenylpyrroles.
Table 1.
Fungicides with actions similar to fludioxonil
| Class of fungicide | Examples | Mode of action | Notes |
|---|---|---|---|
| Phenylpyrrole | Fludioxonil, Fenpiclonil | Inhibition of respiration in mitochondria | Requires HOG1 pathway |
| Dicarboxamide | Procymidone, Vinclozolin | Inhibition of respiration in mitochondria | Induces ROS |
| Carboxamide | Caboxin, Boscalid, Flutolanil | Inhibition of respiration in mitochondria | Elevates NO in plants |
| Qols (Strobilurin) | Azoxystrobin, Fenamidone | Inhibition of respiration in mitochondria | Elevates NO in plants |
| Chloronitriles/Phthalonitriles | Chlorothalonil | Inhibition of respiration in mitochondria | Depletes GSH, reaction with thiols |
| 2,6-dinitroanilines | Fluazinam | Inhibition of respiration in mitochondria | reaction with thiols |
| Cyanoimidizole | Cyazofamid | Inhibition of respiration in mitochondria | |
| Polyketide | Ambruticin | Acts on HOG1 pathway(?) | |
| Aromatic hydrocarbon | Pentachloronitro-benzene (PCNB) | Induces ROS |
History
Researchers from Eli Lilly first isolated pyrrolnitrin from Pseudomonas pyrrocinia in 1964. This molecule, metabolized from tryptophan, was found to have anti-fungal properties, but was not considered viable as a pesticide due to photochemical instability (Arima et al., 1964; Arima et al., 1965).
The mechanism by which pyrrolnitrin inhibited cellular function was reported at that time to be inhibition of the mitochondrial electron transport chain, predominantly impacting respiration of mitochondria at complex I (Wong and Airall, 1970; Wong, Horng and Gordee, 1971). Inhibition at this complex can cause electrons to “short-circuit” to molecular oxygen, generating the damaging reactive oxygen species (ROS) superoxide (Murphy, 2009). Though primarily investigated with respect to fungicidal applications, this tendency to induce ROS was described first in mammalian cells (Coleman et al., 2012) and in isolated mitochondria (Syromyatnikov et al., 2017; Wong and Airall, 1970).
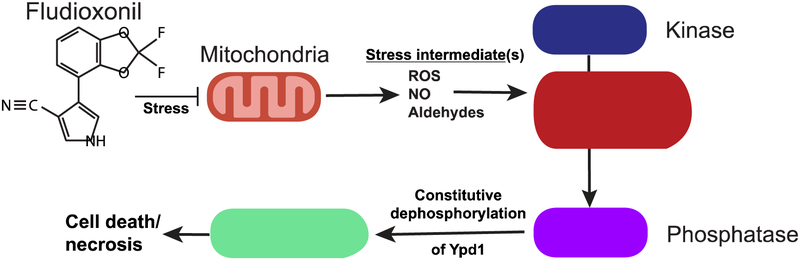
In 1993, fludioxonil was engineered by Ciba-Geigy (now Syngenta). Their intention was never to alter its mode of action, but rather to amend pyrrolnitrin’s tendency to photodecay rapidly in the environment (Leadbitter, 1994). In this they were successful: fludioxonil was both incredibly hydrophobic and unreactive, persisting for weeks after application (Figure 1). The strength of its anti-fungal effect was undiminished.
Figure 1. Theoretical mechanism of fludioxonil action.
We postulate that instead of acting directly upon a prototypical hybrid histidine kinase (HHK), fludioxonil instead creates a stress state in affected cells. Stress intermediates derived from this state are then detected via their modification of sentinel cysteines present in the HHK, inducing a structural shift that favors the phosphatase activity of the HHK over the kinase activity. Dephosphorylation of Ypd1 directly activates the HOG1 cascade, interfering with cell cycle and glycerol production pathways and swiftly rendering the cell non-viable. This model is based in part on published data (Lawry et al., 2017).
In 1997, Ciba-Geigy first proposed that accumulation of cellular glycerol was responsible for the mechanism of fludioxonil action, and outlined its ability to trigger the HOG osmoregulation pathway, which does not have an analog in animals (Pillonel and Meyer, 1997). Solid proof for this conjecture was not forthcoming, but sensitive yeast cells were seen to shed quantities of glycerol upon lysis. This mode of action was touted as evidence that fludioxonil posed little risk to the health of off-target organisms, and the initial reports supporting such a fungus-specific mechanism are likely to have figured into the EPA’s acceptance of this product, the proliferation of novel, prescribed applications and the commensurate demand.
Ongoing research into fludioxonil, however, uncovered flaws in the mechanism proffered by Ciba-Geigy/Syngenta. In 2007 it was proven that disruption of glutathione (GSH) homeostasis (which serves to buffer nitrosative, oxidative and aldehydic stressors) in fungi synergistically enhances the activity of fludioxonil. This suggested that damage derived from these stressors, or the fungal response to them, may figure prominently in the mechanism of fludioxonil toxicity, possibly overshadowing an osmoregulation mechanism (Kim et al., 2007b, 2007a). In 2008, it was shown that the osmoregulation pathway of Botrytis cinerea was specifically dispensable with regard to its sensitivity to fludioxonil. The fungus was seen to retain sensitivity in isolates from which this pathway had been deleted (Liu, Leroux and Fillinger, 2008). Further, fludioxonil activity is synergistically enhanced by compounds that interfere with mitochondrial respiration and anti-oxidation systems, while overproduction of elements that suppress such damage substantially inhibit activity. These findings seem to suggest that the original mechanism of activity determined for pyrrolnitrin in 1970 may remain relevant to the drug action of fludioxonil today, and stress intermediates, oxidative or otherwise, may be a factor in that activity (Kim et al., 2010).
A study by Upadya in 2013 invalidated claims that fludioxonil killed fungi by causing the overproduction of glycerol. In C. neoformans and B. cinera, glycerol content was identical between sensitive and resistant isolates treated with fludioxonil. While elements of the osmoregulation pathway may be activated by fludioxonil, overproduction of glycerol is not requisite for the sensitivity of fungi to this fungicide (Upadhya et al., 2013; Li et al., 2014).
Finally, in 2016, Lawry et. al. demonstrated that fludioxonil activates the HOG pathway by suppressing the kinase activity of a group III HHK, causing it to convert into a phosphatase. This drug effect could not be triggered directly in vitro using the purified HHK, however, suggesting that fludioxonil might act indirectly through an as yet uncharacterized upstream target (Lawry et al., 2017). HHKs often serve as sensor kinases with especially sensitive sentinel cysteine thiols reacting to elevations of reactive stress molecules such as ROS, nitric oxide (NO) or aldehydic stressors (derived from lipid oxidation or glycolytic imbalance)(Wong, Chen and Gan, 2015; Hancock et al., 2006). If the HHKs governing fungal HOG pathways are responding to an increase in stress molecules, it means that fludioxonil exercises its action by induction of these stress molecules in target fungal pathogens (Figure 1). It remains to be determined whether this previously unappreciated cellular stress might pose a risk of toxicity to non-target organisms and cells. Evidence for such adverse health effects would certainly call into question the presumptions that fostered the wide-spread acceptance of fludioxonil for use on fruits and vegetables, especially in post-harvest applications.
Evidence for adverse health effects
In 2012, human glial cells and neuronal cells incubated with fludioxonil showed losses in membrane potential and ATP production at concentrations well below those considered toxic. Cellular thiol levels dropped and GSH peroxidase and superoxide dismutase were transcriptionally induced (Coleman et al., 2012)- all characteristic indicators of severe oxidative damage. A year later, toxicologists characterized fludioxonil as an endocrine disruptor in human breast cancer cells. It also impacted their overall viability and proliferation though an undetermined mechanism (Teng et al., 2013). During that same year, NADPH oxidase mutants of Alternaria alternata that were resistant to fludioxonil were found to be similarly resistant to the dicarboximide fungicide vinclozolin (Yang and Chung, 2013). This raises the possibility that these fungicides may operate through the same pathway. Authors speculated this pathway was either respondent to oxidative insult or acted to produce it. This is especially relevant here because vinclozolin was established to be an anti-androgen endocrine disruptor (Gray, Ostby and Kelce, 1994) in 1994, and its use has been disallowed for most applications in the US since 2003 (van Ravenzwaay et al., 2013). The capacity for vinclozolin to induce endocrine abnormalities is now well accepted, with evidence of accompanying oxidative stress detected in vivo (Reddy, 2014).
When the pesticide fludioxonil first went through regulatory review by the EPA, it was posited that fludioxonil acted primarily through activation of the HOG1 osmoregulation pathway by direct interaction with fUngal-specific HHKs. This mechanism, unchallenged at the time, argued for its safety- comparable perhaps to antibiotics that specifically target bacterial cell walls. As this relatively benign mechanism has now been called into question, it may be prudent to reconsider a series of reports claiming fludioxonil exposure was harmful in non-target organisms. Evidence seems to suggest that part of the mechanism involves or causes disruption of electron transport (respiration) in mitochondria, the mode of action advanced for pyrrolnitrin back in 1972 (Lambowitz and Slayman, 1972). Inhibition of electron transport at complex 1 is known to catalyze the creation of superoxide, which is swiftly converted by superoxide dismutase (SOD) to hydrogen peroxide. As fludioxonil has been shown to inhibit catalase, the normal means by which cells neutralize peroxide, H2O2 might be expected to become elevated in the cytosol (Karadag and Ozhan, 2015). Whether ROS represent the direct mechanism of fludioxonil action or a side-effect, accumulation of these damaging molecules in any cell type is undesireable, with potential to cause single- and double-strand breaks in DNA and inactivate critical enzymes (Ojha and Srivastava, 2014).
In mammals, the liver absorbs and neutralizes many contaminants that could threaten cellular processes. Strongly hydrophobic molecules introduced into the GI tract are rendered soluble by bile salts during digestion and thus they are delivered into the hepatobiliary system (Moghimipour, Ameri and Handali, 2015). This is relevant to fludixonil toxicity because the modifications made to pyrrolnitrin to stabilize this pesticide also rendered it exceptionally hydrophobic. Aside from the potential for damage to the liver due to an induced, chronic stress state, prolonged stress to hepatocytes can translate into stress signals like nitric oxide (NO) and methyglyoxal (MG) being exported body-wide (Xinyun Xu, 2010; Kuo, Abe and Schroeder, 1997; Akaike, 2000). This could contribute to a variety of disease states, dependent upon a similarly extensive variety of genetic variations in overall and organ-specific responses to oxidative stress. The number of diseases that are co-morbid with elevated NO alone, including autism (Sweeten et al., 2004), rheumatoid arthritis, diabetes, inflammatory bowel disease, and multiple sclerosis (Parkinson, Mitrovic and Merrill, 1997)) is substantial and worthy of consideration.
In 2016, the EPA’s own ToxCast “toxicity forecaster” screen, which uses high-throughput bioassays to evaluate potentially toxic side effects of environmentally-relevant small molecules (Shah et al., 2016; Kavlock et al., 2012), identified fludioxonil as an inducer of oxidative damage by measurements of mitochondrial mass and histone phosphorylation in hepatocytes. Some researchers have begun to question the safety of fludioxonil as evidence of oxidative stress and endocrine disruption accrues. Mere confirmation of these phenomena does not inform us regarding the actual mechanism by which fludioxonil acts, however, and this piece of the puzzle needs to fall into place to fully understand the impact this pesticide may have on human health.
Recent discoveries germane to pesticide toxicity
In 2014, inhibition of mitochondrial respiration at complex I was identified as the likely cause of neurotoxicity in chlorpyrifos, an organophosphate insecticide (Salama et al., 2014). Two years later, a decade long study by researchers at UCLA established that organophosphate insecticides could be causally linked to Parkinson’s disease in individuals with certain (not uncommon) neuronal Nitric Oxide Synthase gene variants (Paul et al., 2016). Thus, in an organophosphate pesticide, environmental exposure is thought to suppress mitochondrial respiration, and in humans with specific gene variants this demonstrably leads to one of many neurological diseases with a previously unknown cause. This case may not be isolated. Oxidative stress has been known to be a mechanism of toxicity in many pesticides since at least 2004, and the implication of oxidative, nitrosative and aldehydic stresses in an impressive list of ailments and disorders has been deduced as well (Abdollahi et al., 2004). (eg: lupus, COPD (Ryan, Nissim and Winyard, 2014), and Parkinson’s disease (Hwang, 2013)).
The induction of oxidative stress by pesticides has been demonstrated numerous times, most recently in the pesticides Maneb and Paraquat (Shukla et al., 2015). It has been argued that excessive NO could represent a symptom rather than a cause of diseases, but in MRL-lpr/lpr mice, which are naturally very prone to autoimmune dysfunction (Gu et al., 1998), elevated NO precedes the development of any such disease states. Furthermore, if you inhibit the production of excessive NO in these mice by inhibiting NO synthase, this prevents or at least attenuates the disease states that they normally develop (Gilkeson et al., 1997). This suggests that oxidative/nitrosative stress could be a precursor for any number of autoimmune endocrine disorders, neurological disorders, or inflammatory disorders, possibly dependent on genetic factors that render certain subpopulations at greater risk, yet not so much so that these genes would be considered causal.
The aggregative impact of synergistic toxins
That the toxic impact of pesticides hinges upon the dosage absorbed is undeniable. This point is echoed and emphasized by essentially every producer of pesticide chemicals. Unfortunately, pesticide toxicity testing remains, largely, piecemeal, and seldom are synergy or aggregate toxicity considered. This may be a critical oversight. Further, measurements of toxicity often focus solely upon lethal dosages and carcinogenesis. Damage to the body’s capacity to manage oxidative, nitrosative, or aldehydic stress has only recently entered into considerations of toxicity, and there almost exclusively with pharmaceuticals.
These two factors may be of immediate concern considering that a product applied extensively to com and soy crops, Roundup, has been demonstrated to impact mitochondrial respiration as well (Peixoto, 2005). Imidicloprid, a popular insecticide, has been found to induce NO production in brain, liver and nerve cells (Duzguner and Erdogan, 2012) - a fact that should be concerning. Even if we ignore these major players in the agrochemical world, and focus upon fungicides alone, we discover that there are literally dozens that share elements of their putative mode of action with fludioxonil (Table 1). Moreover, many fungicides are known to be more effective when applied alongside pesticides that deplete anti-oxidant capacity or that disable stress response pathways (Kim et al., 2007a, 2007b), so concern over the possiblility of synergistic exacerbation of toxicity does not seem groundless.
For decades there has been debate regarding the risks posed by pesticides in our food supply. Despite reports finding no difference in nutrition between organic and non-organic foods, or downplaying health consequences of pesticides, the latest reports describe an impact upon human health such that an organic diet promotes optimal health status and decreases the risk of developing chronic disease (Hurtado-Barroso et al., 2017). Perhaps, with this in mind, it is time to exercise the same level of vigilance we apply to pharmaceuticals to those fungicides that are liberally applied to our food post-harvest. At the very least, an effort to clarify specific mechanisms of widely used pesticides seems in order, such that the most dangerous of these toxins may be limited or removed from our food stream. In place of dangerous post-harvest fungicides, safe and edible polysaccharide coatings have recently been developed that seal and protect food surfaces from fungal infection, and these would seem to be a realistic, healthier alternative. (Hassan et al., 2018)
Supplementary Material
Highlights.
The mechanism by which fludioxonil kills fungi remains unknown.
The theoretical mechanism, long supported by the agrochemical industry and safe for off-target organisms, has been called into question by recent research.
The action of fludioxonil is associated with indicators of severe oxidative stress and has been linked to disruption of endocrine processes.
Many pesticides may act along analogous pathways, leading to a strong potential for aggregative or synergistic toxicity, with potential to impact human health.
Acknowledgements
This work benefitted from efforts by Laura J. Roelans searching pesticide and patent databases (within NIH, CDC, FDA and EPA not to mention European Union) as well as providing insightful discussion. The authors are supported by USPHS NIH grant R21 AI123758.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, and Rezaie A. (2004). Pesticides and oxidative stress: a review, Med Sci Monit 10:RA141–7. [PubMed] [Google Scholar]
- Akaike T (2000). Mechanisms of biological S-nitrosation and its measurement, Free Radic Res 33:461–9. [DOI] [PubMed] [Google Scholar]
- Arima K, Imanaka H, Kousaka M, Fukuda A, and Tamura G. (1965). Studies on pyrrolnitrin, a new antibiotic. I. Isolation and properties of pyrrolnitrin, J Antibiot (Tokyo) 18:201–4. [PubMed] [Google Scholar]
- Arima Kei, Imanaka Hiroshi, Kousaka Masanobu, Fukuta Akio, and Tamura Gakuzo. (1964). Pyrrolnitrin, a New Antibiotic Substance, Produced by Pseudomonas, Agricultural and Biological Chemistry 28:575–76. [Google Scholar]
- Code of Federal Regulations for Fludioxonil. (2005). Environmental Protection Agency. [Google Scholar]
- Coleman MD, O'Neil JD, Woehrling EK, Ndunge OB, Hill EJ, Menache A, and Reiss CJ. (2012). A preliminary investigation into the impact of a pesticide combination on human neuronal and glial cell lines in vitro, PLoS One 7:e42768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzguner V, and Erdogan S. (2012). Chronic exposure to imidacloprid induces inflammation and oxidative stress in the liver & central nervous system of rats, Pesticide Biochemistry and Physiology 104:58–64. [Google Scholar]
- Furukawa K, Randhawa A, Kaur H, Mondal AK, and Hohmann S. (2012). Fungal fludioxonil sensitivity is diminished by a constitutively active form of the group III histidine kinase, FEBS Lett. [DOI] [PubMed] [Google Scholar]
- Gehmann K, Nyfeler R, Leadbeater AJ, Nevill DJ, and Sozzi D. (1990). CGA 173506: a new phenylpyrrole fungicide for broad-spectrum disease control. In Brighton Crop Protection Conference, Pests and Diseases - 1990. Vol. 2. [Google Scholar]
- Gilkeson GS, Mudgett JS, Seldin MF, Ruiz P, Alexander AA, Misukonis MA, Pisetsky DS, and Weinberg JB. (1997). Clinical and serologic manifestations of autoimmune disease in MRL-lpr/lpr mice lacking nitric oxide synthase type 2, J Exp Med 186:365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE Jr., Ostby JS, and Kelce WR. (1994). Developmental effects of an environmental antiandrogen: the fungicide vinclozolin alters sex differentiation of the male rat, Toxicol Appl Pharmacol 129:46–52. [DOI] [PubMed] [Google Scholar]
- Gu L, Weinreb A, Wang XP, Zack DJ, Qiao JH, Weisbart R, and Lusis AJ. (1998). Genetic determinants of autoimmune disease and coronary vasculitis in the MRL-lpr/lpr mouse model of systemic lupus erythematosus, J Immunol 161:6999–7006. [PubMed] [Google Scholar]
- Hancock John, Desikan Radhika, Harrison Judith, Bright Jo, Hooley Richard, and Neill Steven. (2006). Doing the unexpected: proteins involved in hydrogen peroxide perception, J Exp Bot 57:1711–18. [DOI] [PubMed] [Google Scholar]
- Hassan B, Chatha SAS, Hussain AI, Zia KM, and Akhtar N. (2018). Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review, Int J Biol Macromol 109:1095–107. [DOI] [PubMed] [Google Scholar]
- Hurtado-Barroso S, Tresserra-Rimbau A, Vallverdu-Queralt A, and Lamuela-Raventos RM. (2017). Organic food and the impact on human health, Crit Rev Food Sci Nutr:1–11. [DOI] [PubMed] [Google Scholar]
- Hwang O (2013). Role of oxidative stress in Parkinson's disease, Exp Neurobiol 22:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespers ABK, and Dewaard MA. (1995). Effect of Fenpiclonil on Phosphorylation of Glucose in Fusarium-Sulphureum, Pesticide Science 44:167–75. [Google Scholar]
- Karadag H, and Ozhan F. (2015). Effect of cyprodinil and fludioxonil pesticides on bovine liver catalase activity, Biotechnol Biotechnol Equip 29:40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock R, Chandler K, Houck K, Hunter S, Judson R, Kleinstreuer N, Knudsen T, Martin M, Padilla S, Reif D, Richard A, Rotroff D, Sipes N, and Dix D. (2012). Update on EPA's ToxCast program: providing high throughput decision support tools for chemical risk management, Chem Res Toxicol 25:1287–302. [DOI] [PubMed] [Google Scholar]
- Kilani J, and Fillinger S. (2016). Phenylpyrroles: 30 Years, Two Molecules and (Nearly) No Resistance, Front Microbiol 7:2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Campbell BC, Mahoney N, Chan KL, Molyneux RJ, and May GS. (2007a). Enhanced activity of strobilurin and fludioxonil by using berberine and phenolic compounds to target fungal antioxidative stress response, Lett Appl Microbiol 45:134–41. [DOI] [PubMed] [Google Scholar]
- ———. (2007b). Enhancement of fludioxonil fungicidal activity by disrupting cellular glutathione homeostasis with 2,5-dihydroxybenzoic acid, FEMS Microbiol Lett 270:284–90. [DOI] [PubMed] [Google Scholar]
- Kim JH, Campbell BC, Mahoney N, Chan KL, Molyneux RJ, and Xiao CL. (2010). Use of chemosensitization to overcome fludioxonil resistance in Penicillium expansum, Lett Appl Microbiol 51:177–83. [DOI] [PubMed] [Google Scholar]
- Kojima K, Takano Y, Yoshimi A, Tanaka C, Kikuchi T, and Okuno T. (2004). Fungicide activity through activation of a fungal signalling pathway, Mol Microbiol 53:1785–96. [DOI] [PubMed] [Google Scholar]
- Kuo PC, Abe KY, and Schroeder RA. (1997). Oxidative stress increases hepatocyte iNOS gene transcription and promoter activity, Biochem Biophys Res Commun 234:289–92. [DOI] [PubMed] [Google Scholar]
- Lambowitz AM, and Slayman CW. (1972). Effect of pyrrolnitrin on electron transport and oxidative phosphorylation in mitochondria isolated from Neurospora crassa, J Bacteriol 112:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawry SM, Tebbets B, Kean I, Stewart D, Hetelle J, and Klein BS. (2017). Fludioxonil Induces Drk1, a Fungal Group III Hybrid Histidine Kinase, To Dephosphorylate Its Downstream Target, Ypd1, Antimicrob Agents Chemother 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbitter NJ, Nyfeler R, and Elmsheuser H (1994). “The phenylpyrroles: thehistory of their development at Ciba,”. In, 129–34. University of Kent, Canterbury. [Google Scholar]
- Lew RR (2010). Turgor and net ion flux responses to activation of the osmotic MAP kinase cascade by fludioxonil in the filamentous fungus Neurospora crassa, Fungal Genet Biol 47:721–6. [DOI] [PubMed] [Google Scholar]
- Li X, Fernandez-Ortuno D, Grabke A, and Schnabel G. (2014). Resistance to fludioxonil in Botrytis cinerea isolates from blackberry and strawberry, Phytopathology 104:724–32. [DOI] [PubMed] [Google Scholar]
- Liu W, Leroux P, and Fillinger S. (2008). The HOG1-like MAP kinase Sak1 of Botrytis cinerea is negatively regulated by the upstream histidine kinase Bos1 and is not involved in dicarboximide- and phenylpyrrole-resistance, Fungal Genet Biol 45:1062–74. [DOI] [PubMed] [Google Scholar]
- Moghimipour E, Ameri A, and Handali S. (2015). Absorption-Enhancing Effects of Bile Salts, Molecules 20:14451–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama T, Kadokura K, Ohira T, Ichiishi A, Fujimura M, Yamaguchi I, and Kudo T. (2005). A two-component histidine kinase of the rice blast fungus is involved in osmotic stress response and fungicide action, Fungal Genet Biol 42:200–12. [DOI] [PubMed] [Google Scholar]
- Motoyama T, Ohira T, Kadokura K, Ichiishi A, Fujimura M, Yamaguchi I, and Kudo T. (2005). An Os-1 family histidine kinase from a filamentous fungus confers fungicide-sensitivity to yeast, Current genetics 47:298–306. [DOI] [PubMed] [Google Scholar]
- Murphy MP (2009). How mitochondria produce reactive oxygen species, Biochem J 417:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai N, Fujimura M, Motoyama T, Ichiishi A, Usami R, Horikoshi K, and Yamaguchi I. (2001). Characterization of mutations in the two-component histidine kinase gene that confer fludioxonil resistance and osmotic sensitivity in the os-1 mutants of Neurospora crassa, Pest Manag Sci 57:437–42. [DOI] [PubMed] [Google Scholar]
- Ojha A, and Srivastava N. (2014). In vitro studies on organophosphate pesticides induced oxidative DNA damage in rat lymphocytes, Mutat Res Genet Toxicol Environ Mutagen 761:10–7. [DOI] [PubMed] [Google Scholar]
- Parkinson JF, Mitrovic B, and Merrill JE. (1997). The role of nitric oxide in multiple sclerosis, J Mol Med (Berl) 75:174–86. [DOI] [PubMed] [Google Scholar]
- Paul KC, Sinsheimer JS, Rhodes SL, Cockburn M, Bronstein J, and Ritz B. (2016).Organophosphate Pesticide Exposures, Nitric Oxide Synthase Gene Variants, and Gene-Pesticide Interactions in a Case-Control Study of Parkinson's Disease, California (USA), Environ Health Perspect 124:570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto F (2005). Comparative effects of the Roundup and glyphosate on mitochondrial oxidative phosphorylation, Chemosphere 61:1115–22. [DOI] [PubMed] [Google Scholar]
- Pillonel C, and Meyer T. (1997). Effect of phenylpyrroles on glycerol accumulation and protein kinase activity of Neurospora crassa, Pesticide Science 49:229–36. [Google Scholar]
- Reddy B. Bhavanarayana and Sreenivasula. P (2014). Elevation of oxidative stress in the testes of rat by vinclozolin, International Journal of Pharm Tech Research 6:363–68. [Google Scholar]
- Ryan BJ, Nissim A, and Winyard PG. (2014). Oxidative post-translational modifications and their involvement in the pathogenesis of autoimmune diseases, Redox Biol 2:715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama M, El-Morsy D, El-Gamal M, Shabka O, and Mohamed WM. (2014). Mitochondrial complex I inhibition as a possible mechanism of chlorpyrifos induced neurotoxicity, Ann Neurosci 21:85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah I, Setzer RW, Jack J, Houck KA, Judson RS, Knudsen TB, Liu J, Martin MT, Reif DM, Richard AM, Thomas RS, Crofton KM, Dix DJ, and Kavlock RJ. (2016). Using ToxCast™ Data to Reconstruct Dynamic Cell State Trajectories and Estimate Toxicological Points of Departure, Environ Health Perspect 124:910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S, Singh D, Kumar V, Chauhan AK, Singh S, Ahmad I, Pandey HP, and Singh C. (2015). NADPH oxidase mediated maneb- and paraquat-induced oxidative stress in rat polymorphs: Crosstalk with mitochondrial dysfunction, Pestic Biochem Physiol 123:74–86. [DOI] [PubMed] [Google Scholar]
- Sweeten TL, Posey DJ, Shankar S, and McDougle CJ. (2004). High nitric oxide production in autistic disorder: a possible role for interferon-gamma, Biol Psychiatry 55:434–7. [DOI] [PubMed] [Google Scholar]
- Syromyatnikov MY, Kokina AV, Lopatin AV, Starkov AA, and Popov VN. (2017). Evaluation of the toxicity of fungicides to flight muscle mitochondria of bumblebee (Bombus terrestris L.), Pestic Biochem Physiol 135:41–46. [DOI] [PubMed] [Google Scholar]
- Teng Y, Manavalan TT, Hu C, Medjakovic S, Jungbauer A, and Klinge CM. (2013). Endocrine disruptors fludioxonil and fenhexamid stimulate miR-21 expression in breast cancer cells, Toxicol Sci 131:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhya R, Kim H, Jung KW, Park G, Lam W, Lodge JK, and Bahn YS. (2013). Sulphiredoxin plays peroxiredoxin-dependent and -independent roles via the HOG signalling pathway in Cryptococcus neoformans and contributes to fungal virulence, Mol Microbiol 90:630–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ravenzwaay B, Kolle SN, Ramirez T, and Kamp HG. (2013). Vinclozolin: a case study on the identification of endocrine active substances in the past and a future perspective, Toxicol Lett 223:271–9. [DOI] [PubMed] [Google Scholar]
- Vetcher L, Menzella HG, Kudo T, Motoyama T, and Katz L. (2007). The antifungal polyketide ambruticin targets the HOG pathway, Antimicrob Agents Chemother 51:3734–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DT, and Airall JM. (1970). The mode of action of antifungal agents: effect of pyrrolnitrin on mitochondrial electron transport, J Antibiot (Tokyo) 23:55–62. [DOI] [PubMed] [Google Scholar]
- Wong DT, Horng JS, and Gordee RS. (1971). Respiratory chain of a pathogenic fungus, Microsporum gypseum: effect of the antifungal agent pyrrolnitrin, J Bacteriol 106:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Chen Y, and Gan YH. (2015). Host Cytosolic Glutathione Sensing by a Membrane Histidine Kinase Activates the Type VI Secretion System in an Intracellular Bacterium, Cell Host Microbe 18:38–48. [DOI] [PubMed] [Google Scholar]
- Xu Xinyun, Wu Peiqiao, Ke Yuebin, Zhou Li, He Cai, Yuan Jianhui & Huang Haiyan. (2010). Oxidative stress and inducible nitric oxide synthase expression in human hepatocytes treated with trichloroethylene, Toxicological & Environmental Chemistry 92. [Google Scholar]
- Yang SL, and Chung KR. (2013). Similar and distinct roles of NADPH oxidase components in the tangerine pathotype of Alternaria alternata, Mol Plant Pathol 14:543–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimi A, Kojima K, Takano Y, and Tanaka C. (2005). Group III histidine kinase is a positive regulator of Hog1-type mitogen-activated protein kinase in filamentous fungi, Eukaryotic cell 4:1820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.