The frequency of viral respiratory pathogens in asymptomatic subjects is poorly defined. The aim of this study was to explore the prevalence of respiratory pathogens in the upper airways of asymptomatic adults, compared with a reference population of symptomatic patients sampled in the same centers during the same period.
KEYWORDS: respiratory pathogens, respiratory viruses, virology
ABSTRACT
The frequency of viral respiratory pathogens in asymptomatic subjects is poorly defined. The aim of this study was to explore the prevalence of respiratory pathogens in the upper airways of asymptomatic adults, compared with a reference population of symptomatic patients sampled in the same centers during the same period. Nasopharyngeal (NP) swab samples were prospectively collected from adults with and without ongoing symptoms of respiratory tract infection (RTI) during 12 consecutive months, in primary care centers and hospital emergency departments, and analyzed for respiratory pathogens by a PCR panel detecting 16 viruses and four bacteria. Altogether, 444 asymptomatic and 75 symptomatic subjects completed sampling and follow-up (FU) at day 7. In the asymptomatic subjects, the detection rate of viruses was low (4.3%), and the most common virus detected was rhinovirus (3.2%). Streptococcus pneumoniae was found in 5.6% of the asymptomatic subjects and Haemophilus influenzae in 1.4%. The only factor independently associated with low viral detection rate in asymptomatic subjects was age ≥65 years (P = 0.04). An increased detection rate of bacteria was seen in asymptomatic subjects who were currently smoking (P < 0.01) and who had any chronic condition (P < 0.01). We conclude that detection of respiratory viruses in asymptomatic adults is uncommon, suggesting that a positive PCR result from a symptomatic patient likely is relevant for ongoing respiratory symptoms. Age influences the likelihood of virus detection among asymptomatic adults, and smoking and comorbidities may increase the prevalence of bacterial pathogens in the upper airways.
INTRODUCTION
The detection of respiratory viruses in airway samples by real-time PCR enables early and accurate etiologic diagnosis in respiratory tract infections (RTI). However, the results generated by this sensitive technique can be difficult to interpret. Detection may represent prolonged shedding of virus after symptomatic infection or an asymptomatic infection. Thus, it is essential to evaluate the clinical relevance of a positive finding. Early studies on the prevalence of respiratory viruses in asymptomatic individuals had limited sample sizes and focused on children (1–5). The detection rate of respiratory viruses in asymptomatic children exceeds 30% in some reports (6, 7), with even higher rates in infants (8). The prevalence in asymptomatic adults seems to be lower, ranging from 2.1% to 7.1%, but the number of studies in this field is still limited (9, 10). Different definitions of asymptomatic cases can also affect the reported detection rates. Human rhinovirus (RV) has been the predominant finding in samples from the upper airways in studies of healthy adults. RV detection in asymptomatic persons may occur due to prolonged virus shedding after recovery from a symptomatic illness or to subclinical infection, but reinfection with different serotypes may also explain repeated findings (3, 11–13). Streptococcus pneumoniae and Haemophilus influenzae are important respiratory pathogens that can be cultured in nasopharyngeal samples in asymptomatic individuals with varied frequencies between age groups, representing asymptomatic carriage (14). Detection of these pathogens in the nasopharynx by PCR probably has higher sensitivity than that of conventional cultures, but further studies in healthy adults are needed to evaluate the clinical relevance of detection with this technique for respiratory tract infection and/or asymptomatic carriage (14–19).
The aims of the present study were to determine the prevalence of respiratory pathogens in the upper airways of asymptomatic adults compared with a reference population of patients with respiratory tract infection, and to investigate risk factors associated with the detection of respiratory pathogens in these populations.
MATERIALS AND METHODS
Study outline.
Nasopharyngeal (NP) swab samples were prospectively collected from adults with and without ongoing symptoms of RTI during 12 consecutive months (June 2015 to June 2016). A specially trained study nurse performed sampling and collection of baseline data. The study subjects were recruited from three different primary care centers and three hospital emergency inpatient wards at a 2,000-bed teaching hospital in western Sweden. Inclusion of study subjects was made by the study nurse, who made one or two recruitment visits at each study site (inpatient and primary health care) per week across the entire study period. Asymptomatic subjects were recruited among patients seeking primary health care for, or being admitted to hospital care for, reasons unrelated to respiratory tract infections (e.g., blood pressure controls, annual health check-ups, or lower urinary tract infection in primary care, or minor stroke or ischemic heart disease for hospitalized inpatients). Symptomatic patients in primary health care and inpatient hospital care were recruited as a reference population among patients seeking health care for, or being admitted to hospital for, symptomatic respiratory tract infections. Clinical and laboratory data were recorded in a web-based case report form (CRF), which constituted the study database. The study was approved by the regional ethical review board in Gothenburg, Sweden. All participants provided written informed consent.
Asymptomatic study subjects.
Inclusion criteria for asymptomatic subjects were age ≥18 years and absence of symptoms consistent with RTI during the 2 weeks before enrollment. An NP swab sample was collected (FLOQSwabs; Copan Industries, Inc.), and all participants completed a standardized study-specific questionnaire on demographic and medical data. At 1 week postenrollment, asymptomatic study subjects underwent a telephone-based interview regarding the development of any symptoms of RTI within 4 days after sampling. Individuals who were unable to accurately provide a history, developed symptoms of RTI within 4 days after enrollment, had a history of fever, diarrhea, or antibiotic treatment in the preceding 2 weeks, or resided in a health care facility (e.g., nursing home or residential home) were excluded from participating.
Reference population of symptomatic subjects.
Inclusion criteria were age ≥18 years and symptoms consistent with RTI, as defined below, for a duration of ≤10 days. To distinguish between upper respiratory tract infection (URTI) and lower respiratory tract infection (LRTI), we adapted the definition of URTI from the Wisconsin Upper Respiratory Symptom Survey (WURSS) (20, 21), i.e., at least 1 out of 4 symptoms (nasal discharge, nasal obstruction, sneezing, and/or sore throat) and at least two of the following: sneezing, headache, malaise, chilliness, nasal discharge, nasal obstruction, sore throat, or cough. LRTI was defined according to the Joint Taskforce of the European Respiratory Society (ERS) and European Society for Clinical Microbiology and Infectious Diseases (ESCMID) as an acute illness, usually with cough as the main symptom and at least one of the following symptoms from the lower respiratory tract: sputum production, dyspnea, wheezing, and/or chest discomfort/pain (22). Exclusion criteria for symptomatic subjects were an inability to provide an accurate history, admission to hospital in the last 10 days, or admission from a health care facility, such as a nursing home or residential home. In addition to the standardized study-specific questionnaire, symptomatic subjects also completed a symptom score questionnaire at enrollment and at follow-up (WURSS score for URTI or the Community Acquired Pneumonia Symptom Questionnaire [CAP-Sym] for LRTI [23]).
PCR detection.
All NP swab samples were transported to the laboratory without delay. The samples were analyzed for presence of respiratory pathogens with an in-house multiplex PCR panel that targets 16 viruses and four bacteria. The panel included influenza A (IFA) and influenza B (IFB) virus, respiratory syncytial virus (RSV), rhinovirus (RV), enterovirus (EV), coronavirus (CoV) of four different types (NL63, OC43, 229E, and HKU1), metapneumovirus (MPV), adenovirus (AdV), parainfluenza virus (PIV) types 1 to 4, and bocavirus (BoV), as well as the bacteria Streptococcus pneumoniae, Haemophilus influenzae, Chlamydophila pneumoniae, and Mycoplasma pneumoniae. Briefly, nucleic acid from a 100-µl specimen was extracted into an elution volume of 100 µl by a MagNA Pure LC robot (Roche Molecular Systems, Mannheim, Germany) using the total nucleic acid protocol, and amplified in an ABI 7900 real-time PCR system (Applied Biosystems, Foster City, CA) in 25-µl reaction volumes. After a reverse transcription step, 45 cycles of two-step PCR were performed. Each sample was amplified in 8 parallel reactions, each containing specific primers and probes for 2 or 3 targets. The method has previously been described in detail (24, 25). The cycle threshold (CT) values of positive reactions were recorded, and a positive reaction with CT value of <40 was considered a detection. In cases with a positive signal for both RV and EV with a cycle difference of <5 cycles, indistinguishable EV/RV was recorded.
Statistical analysis.
The frequency of detection was compared with Pearson chi-square or Fisher’s exact test, as appropriate. Comparisons of CT values and clinical parameters were made with simple linear regression and the Pearson correlation coefficient. Factors associated with detection of virus or bacteria with a P value of <0.2 in univariate comparisons were included in multivariate logistic regression models. P values of <0.05 were considered statistically significant (2-sided). All statistical analyses were made using the SPSS software package version 22.0.0.0 (IBM, Armonk, NY).
RESULTS
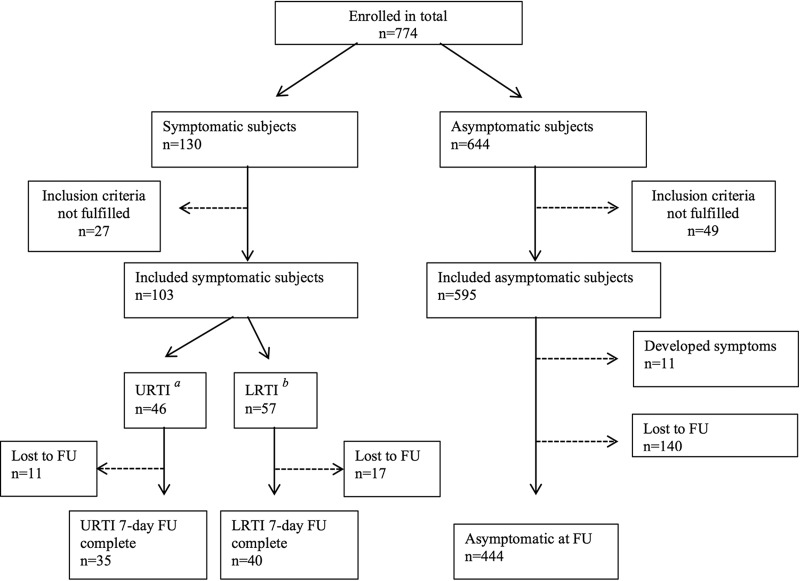
In total, 774 adults agreed to participate in the study. A flowchart of the study subjects is shown in Fig. 1. Altogether, 595 asymptomatic subjects were included, of whom 444 completed follow-up (FU) at day 7. Of the 103 patients with respiratory tract infection who were enrolled in the reference population (46 with URTI and 57 with LRTI), 35 participants with URTI and 40 participants with LRTI completed FU. Demographic data of the included asymptomatic subjects and symptomatic reference patients are presented in Table 1. Monthly seasonal distribution of samples and overall detection rates of respiratory virus and bacteria included in the panel are presented in Table 2.
FIG 1.
Flowchart depicting the enrollment of asymptomatic and symptomatic subjects in the study and subjects lost to follow-up (FU).
TABLE 1.
Demographic data in adults asymptomatic or symptomatic of respiratory tract infectiona
| Characteristicsb | Asymptomatic subjects (n = 444) | Symptomatic reference subjects (n = 103) | P valuec |
|---|---|---|---|
| Age (median [IQR]) (yr) | 66 (57–76) | 69 (54–77) | 0.7 |
| Female sex | 242 (55) | 65 (63) | 0.1 |
| Current smoker | 55 (12) | 21 (20) | 0.03 |
| Chronic lung disease | 66 (15) | 30 (29) | 0.0006 |
| Asthma | 39 (9) | 18 (18) | 0.009 |
| COPD | 20 (5) | 12 (12) | 0.005 |
| Lung cancer | 3 (1) | 1 (1) | 1 |
| Other lung disease | 10 (2) | 7 (7) | 0.03 |
| Any chronic medical conditiond | 286 (64) | 62 (60) | 0.4 |
| Chronic heart disease | 101 (23) | 25 (24) | 0.7 |
| Diabetes mellitus | 96 (22) | 11 (11) | 0.01 |
| Chronic kidney disease | 14 (3) | 1 (1) | 0.3 |
| Chronic liver disease | 4 (1) | 0 | 0.6 |
| Malignancy | 21 (5) | 11 (11) | 0.02 |
| IBD | 12 (3) | 4 (4) | 0.5 |
| Rheumatic disease | 33 (7) | 8 (8) | 0.9 |
| Immunodeficiency | 29 (7) | 6 (6) | 0.8 |
| Other chronic disease | 62 (14) | 9 (9) | 0.2 |
| Children at home | 57 (13) | 6 (6) | 0.04 |
| Children at daycare | 16 (4) | 3 (3) | 1 |
| Influenza vaccination | 166 (37) | 34 (33) | 0.4 |
| Pneumococcal vaccination | 30 (7) | 7 (7) | 1 |
| Antibiotics in last 14 days | 50 (49) | ||
| Duration of symptoms (>7 days) | 30 (29) | ||
| Included at hospital | 97 (22) | 58 (56) | <0.0001 |
| Included at primary health care | 347 (78) | 45 (44) | <0.0001 |
Data presented as number (%), unless otherwise specified.
IQR, interquartile range; IBD, inflammatory bowel disease.
Pearson chi-square (or Fisher’s exact test when appropriate). For age, the Mann-Whitney test was used.
Includes asthma, COPD, lung cancer, and other lung disease.
TABLE 2.
Monthly distribution of total sampling, including number of positive samples for a respiratory virus or bacterium
| Test result by subject group | No. of samples by mo-yr |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jun-15 | July-15 | Aug-15 | Sep-15 | Oct-15 | Nov-15 | Dec-15 | Jan-16 | Feb-16 | Mar-16 | Apr-16 | May-16 | Jun-16 | Total | |
| Asymptomatic subjects | 37 | 2 | 29 | 41 | 39 | 53 | 29 | 30 | 24 | 20 | 39 | 60 | 41 | 444 |
| Positive for respiratory virusa | 1 | 0 | 3 | 4 | 1 | 2 | 3 | 0 | 1 | 0 | 1 | 1 | 2 | 19 |
| Positive for bacteriab | 1 | 1 | 5 | 2 | 1 | 4 | 2 | 4 | 1 | 1 | 4 | 2 | 3 | 31 |
| Symptomatic subjects | 12 | 0 | 4 | 7 | 10 | 11 | 9 | 8 | 3 | 2 | 12 | 19 | 6 | 103 |
| Positive for respiratory virusa | 7 | 0 | 2 | 3 | 2 | 5 | 2 | 4 | 1 | 2 | 3 | 4 | 2 | 37 |
| Positive for bacteriab | 4 | 0 | 2 | 1 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 17 |
All respiratory viruses included in the PCR panel.
Bacteria included in the PCR panel, i.e., Streptococcus pneumoniae and Haemophilus influenzae.
Detection of pathogens in asymptomatic subjects.
Pathogen detection rates are presented in Table 3. A respiratory pathogen was detected in 49 of 444 (11%) asymptomatic subjects. Overall, the detection rate of viruses was low (4.3%). No one in this group had multiple virus findings. The most common virus was RV, followed by CoV. Streptococcus pneumoniae was found in 5.6% of the asymptomatic subjects and Haemophilus influenzae in 1.4%. Of the 140 subjects who did not complete FU, a virus was detected in 5% (n = 3 IFA virus, 2 RV, 1 IFB virus, and 1 CoV) and a bacterium in 5.7% (n = 7 Streptococcus pneumoniae and 1 Haemophilus influenzae). There were no significant differences in the detection rates between the asymptomatic group and the group that was lost to FU. Among the 11 subjects who were excluded due to the development of symptoms within 4 days after inclusion, a virus was detected in 18% (n = 2, both RV). There were no statistically significant differences regarding detection rates between asymptomatic subjects included in primary health care and those in inpatient hospital care (data not shown).
TABLE 3.
Frequency of pathogens detected in the asymptomatic and symptomatic subjects
| Pathogen | No. (%) of subjects |
P valuea | |
|---|---|---|---|
| Asymptomatic (n = 444) | Symptomatic reference subjects (n = 103) | ||
| Any pathogen (including bacteria) | 49 (11) | 51 (50) | <0.0001 |
| Any virus | 19 (4.3) | 37 (36) | <0.001 |
| >1 virus detectedb | 0 | 0 | |
| Rhinovirus | 14 (3.2) | 23 (22) | <0.001 |
| Influenza A virus | 0 | 2 (1.9) | NC |
| Influenza B virus | 0 | 2 (1.9) | NC |
| Coronavirus | 2 (0.5) | 6 (5.8) | 0.0008 |
| Enterovirus | 1 (0.2) | 0 | NC |
| Adenovirus | 0 | 0 | NC |
| Parainfluenzavirus | 0 | 1 (1.0) | NC |
| Bocavirus | 1 (0.2) | 0 | NC |
| RS-virus | 0 | 2 (1.9) | NC |
| Metapneumovirus | 1 (0.2) | 1 (1.0) | NC |
| S. pneumoniae | 25 (5.6) | 7 (6.8) | 0.65 |
| H. influenzae | 6 (1.4) | 10 (9.7) | 0.0001 |
| M. pneumoniae | 0 | 1 (1.0) | NC |
Pearson chi-square (or Fisher’s exact test when appropriate).
NC, not calculated due to small numbers.
Detection of pathogens in reference population of symptomatic subjects.
The detection rate was significantly higher among symptomatic subjects than among asymptomatic subjects (Table 3). Overall, 51 out of 103 (50%) patients with RTI were positive for any respiratory pathogen, and in 37 cases (36%), a virus was detected. No one in this group had multiple virus detections. RV was the predominant virus, followed by CoV. Haemophilus influenzae was significantly more frequent in symptomatic than in asymptomatic subjects, but the frequency of Streptococcus pneumoniae was similar in the two groups. There were no statistically significant differences regarding detection rates between symptomatic subjects included in primary health care and those in inpatient hospital care (data not shown).
Factors associated with detection of virus or bacteria in asymptomatic subjects.
In the univariate model, age ≥65 years and previous vaccination against influenza were factors associated with a low probability of viral detection. In the multivariate model, only age ≥65 years remained independently associated with viral detection. For bacteria, current smoking and the presence of any chronic medical condition were associated with a high probability of detection in both the univariate and multivariate models. No other predictive factors associated with detection of virus or bacteria was identified in asymptomatic subjects or in the reference patients with respiratory symptoms (data not shown).
Viral load and symptom scores.
For the three most common pathogens (RV, Streptococcus pneumoniae, and Haemophilus influenzae), comparisons of pathogen load, as estimated by the CT value, between the asymptomatic and the symptomatic groups were made. A trend toward lower pathogen load was observed in asymptomatic compared with symptomatic subjects, but the difference was not statistically significant for any of the three pathogens (data not shown). Among the 35 symptomatic reference subjects who fulfilled the criteria for URTI according to WURSS score at day 0 and day 7, a virus was detected in 43% (n = 15 [9 RV, 3 CoV, 2 IFA virus, 1 and IFB virus]). Another 40 reference patients fulfilled the criteria for LRTI and completed CAP-Sym at day 0 and day 7. In 17 (43%) cases, a virus was detected (11 RV, 1 IFB virus, 1 CoV, 1 MPV, 1 PIV, and 1 RSV). Thirty-one patients (78%) with LRTI reported treatment with antibiotics in the last 2 weeks compared to 3 (8.6%) cases with URTI (P < 0.01). Further analysis of the WURSS score and CAP-Sym score did not reveal any relevant significant results (data not shown).
DISCUSSION
In this prospective study, we analyzed the prevalence of respiratory viruses by PCR in nasopharyngeal swab samples in a large group of asymptomatic adults sampled across all seasons. The main finding is that the detection rate of respiratory viruses in asymptomatic adults was low (4.3% positive). Only 1% of the participants were positive for other viruses than rhinovirus, which has previously been reported to cause prolonged shedding postinfection. There were no significant differences between samples from primary health care and inpatient samples. In contrast, the viral detection rate was high in the reference population of patients with symptomatic respiratory tract infection, where viruses were found in more than one-third of samples. The results suggest that asymptomatic infections are rare in immunocompetent adult patients and that detection of respiratory viruses in this group in general is clinically relevant. We detected relatively few cases of IFA virus among the symptomatic subjects. The duration of the influenza season was relatively short this year, and healthy subjects are generally advised to avoid seeking care for typical symptoms of uncomplicated seasonal influenza. Furthermore, a relatively large proportion (43%) of the symptomatic subjects age ≥65 years stated having previous vaccination against IFA.
Early reports on the prevalence of virus in subjects without respiratory tract symptoms, summarized in a meta-analysis by Jartti et al., indicated that respiratory viruses were rarely (≤5%) present (although persistence might last up to a few weeks) and, accordingly, that positive results likely reflect recently acquired respiratory infections (26). More recent studies have reported a high frequency of respiratory viruses in asymptomatic children, ranging from 28% to 52% or even higher in infants (8, 27–30), often with predominance of RV (6). Available data indicate that detection rates in asymptomatic adults are much lower. For example, a respiratory virus was detected in 2% of asymptomatic controls in two studies of community-acquired pneumonia (10, 31), observations that agree with our results. Higher rates of virus infections were found by Lieberman et al. in 450 asymptomatic adults (7.1%) and in 201 adults with LRTI without pneumonia (54.7%) (9). Collection of both oropharyngeal swabs and nasopharyngeal washings from each participant, in addition to nasopharyngeal swab samples, might have led to higher detection rates in that study. Moreover, no follow-up was performed, and patients sampled in the presymptomatic phase of an upcoming infection might have been included in the asymptomatic group. Self et al. reported virus detection in 24.5% of patients with community-acquired pneumonia (CAP) compared to 43% in our group with LRTI (10). However, only patients with chest imaging suggestive of pneumonia were enrolled in that study. This might have resulted in a high rate of bacterial infections.
As we have reported earlier, RV is the most frequently detected virus in patients with respiratory symptoms, without marked seasonality in a temperate climate (32). In the present study, RV was the major finding in asymptomatic and in symptomatic adults. In immunocompetent subjects, RV infections and concomitant virus shedding commonly resolve within 1 to 2 weeks, although RV may be detected in subjects without respiratory symptoms (3, 11, 12, 30). Based on the large number of asymptomatic subjects, our data suggest that RV infections are less frequent in adults without symptoms of RTI than what was reported by Granados et al., who found RV in about 8% of asymptomatic students (33). Possibly, regional and age differences account for some of the discrepancy. In previous studies, evidence of prolonged shedding has been described, but other studies have also suggested that early reinfection with other RV serotypes is common (13, 34–36). This might imply that RV can cause subclinical infections, with no or mild symptoms, in adults. In the present study, only one sample per individual was obtained, which does not permit a distinction between prolonged shedding and reinfection with a new virus subtype. This issue, as well as to what extent transmission of virus occurs from asymptomatic individuals, warrants further investigation.
Our study is the first, to our knowledge, to explore risk factors for viral infections, as identified by PCR, in a large cohort of asymptomatic adults. Age ≥65 years was significantly associated with a lower viral detection rate in both univariate and multivariate models. In line with our findings, Graat et al. found RV in 2% of asymptomatic elderly (age >60 years) subjects (37). It is possible that the age group <65 years in our study was more exposed to respiratory viruses through close contact with small children, although we did not find any impact of having children at home or in daycare on viral or bacterial detection. Further studies are warranted to explore the association between viral detection in asymptomatic adults and age.
Cigarette smoking increases the risk for bacterial invasion of the airways through several mechanisms, and pneumococcal disease is common in patients with chronic obstructive pulmonary disorder (COPD) (38–41). In line with this, we found an overall carriage rate of S. pneumoniae in 15% of smokers compared to only 4.5% of nonsmokers. However, it is noteworthy that the detection of this pathogen in smokers may be unrelated to their symptoms and that detection may simply reflect asymptomatic carriage. Pneumococcal colonization in the elderly has been reported to be low (<5%), with detection based on culture methods from nasopharyngeal samples. More recent investigations, based on PCR, have presented considerably higher frequencies in this group (15–18). Our PCR-based finding of 4.1% pneumococcal carriage in adult nonsmokers is lower than in these previous reports, although the oropharyngeal tract was not sampled in our study, which may account for the discrepancy. Detection of H. influenzae was common among reference subjects with respiratory symptoms, but the clinical relevance of this finding is unclear. Earlier studies of cultures of NP samples in healthy adults have found various frequencies of H. influenzae, ranging from 1.1% to 29%. Our findings are in line with those from another Swedish study by Gunnarsson et al. (14). In the study by Rawlings et al. (19), samples were collected through the oral cavity and also excluded healthy subjects who had received antibiotics within 4 weeks before sampling. This could possibly have contributed to the higher detection rates found in their study. Although it may be difficult to compare culture methods and PCR-based techniques, we believe that it is important to present the detection rates of S. pneumoniae and H. influenzae in our study, since there remains a lack of knowledge on how to interpret PCR-based detection of these pathogens in NP samples in relation to respiratory symptoms.
We used the WURSS score and CAP-Sym, both validated questionnaires, for symptom scoring in symptomatic cases (21, 23). Although antibiotic prescription patterns were beyond the scope of this article, we did find that 78% of patients who fulfilled the inclusion criteria for the CAP-Sym had been administered antibiotics in the last 2 weeks, compared to 8.6% of the patients with URTI. We did not, however, identify any significant difference in the rates of viral or bacterial pathogens between patients with URTI or LRTI.
This study has limitations. Although we included a large number of asymptomatic adults, the number of symptomatic cases was limited. A difficulty of including younger symptomatic subjects in primary health care due to work-related schedules might have introduced a selection bias toward older participants during recruitment. Sampling, especially of symptomatic subjects, was uneven across the study period, and our series may not reflect the true incidence of infection with any agent. A limited number of study subjects were included during the peak season for influenza and RSV activity (February and March), which may have contributed to an underestimation of the incidences of these viruses among symptomatic cases. The low rate of viral detection in the asymptomatic group may affect the possibility to draw strong conclusions regarding risk factors for the detection of virus in healthy adults. Further studies are needed to evaluate if the risk factors identified in our study are reproducible in a larger cohort. It is also important to note that bacterial findings in this study are based on PCR, which may differ from culture methods in terms of sensitivity.
In conclusion, detection of respiratory viruses in asymptomatic adults is uncommon. A positive PCR result from a symptomatic patient is likely to be relevant for ongoing respiratory symptoms.
REFERENCES
- 1.Falsey AR, Criddle MC, Walsh EE. 2006. Detection of respiratory syncytial virus and human metapneumovirus by reverse transcription polymerase chain reaction in adults with and without respiratory illness. J Clin Virol 35:46–50. doi: 10.1016/j.jcv.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 2.García-García ML, Calvo C, Pozo F, Pérez-Breña P, Quevedo S, Bracamonte T, Casas I. 2008. Human bocavirus detection in nasopharyngeal aspirates of children without clinical symptoms of respiratory infection. Pediatr Infect Dis J 27:358–360. doi: 10.1097/INF.0b013e3181626d2a. [DOI] [PubMed] [Google Scholar]
- 3.Johnston SL, Sanderson G, Pattemore PK, Smith S, Bardin PG, Bruce CB, Lambden PR, Tyrrell DA, Holgate ST. 1993. Use of polymerase chain reaction for diagnosis of picornavirus infection in subjects with and without respiratory symptoms. J Clin Microbiol 31:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S, Wang L, Fan J, Kraft A, Bose ME, Tiwari S, Van Dyke M, Haigis R, Luo T, Ghosh M, Tang H, Haghnia M, Mather EL, Weisburg WG, Henrickson KJ. 2008. Detection of 11 common viral and bacterial pathogens causing community-acquired pneumonia or sepsis in asymptomatic patients by using a multiplex reverse transcription-PCR assay with manual (enzyme hybridization) or automated (electronic microarray) detection. J Clin Microbiol 46:3063–3072. doi: 10.1128/JCM.00625-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nokso-Koivisto J, Kinnari TJ, Lindahl P, Hovi T, Pitkäranta A. 2002. Human picornavirus and coronavirus RNA in nasopharynx of children without concurrent respiratory symptoms. J Med Virol 66:417–420. doi: 10.1002/jmv.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhedin S, Lindstrand A, Rotzen-Ostlund M, Tolfvenstam T, Ohrmalm L, Rinder MR, Zweygberg-Wirgart B, Ortqvist A, Henriques-Normark B, Broliden K, Naucler P. 2014. Clinical utility of PCR for common viruses in acute respiratory illness. Pediatrics 133:e538–e545. doi: 10.1542/peds.2013-3042. [DOI] [PubMed] [Google Scholar]
- 7.Moe N, Pedersen B, Nordbo SA, Skanke LH, Krokstad S, Smyrnaios A, Dollner H. 2016. Respiratory virus detection and clinical diagnosis in children attending day care. PLoS One 11:e0159196. doi: 10.1371/journal.pone.0159196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen RR, Wieringa J, Koekkoek SM, Visser CE, Pajkrt D, Molenkamp R, de Jong MD, Schinkel J. 2011. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol 49:2631–2636. doi: 10.1128/JCM.02094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lieberman D, Shimoni A, Shemer-Avni Y, Keren-Naos A, Shtainberg R, Lieberman D. 2010. Respiratory viruses in adults with community-acquired pneumonia. Chest 138:811–816. doi: 10.1378/chest.09-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Self WH, Williams DJ, Zhu Y, Ampofo K, Pavia AT, Chappell JD, Hymas WC, Stockmann C, Bramley AM, Schneider E, Erdman D, Finelli L, Jain S, Edwards KM, Grijalva CG. 2016. Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J Infect Dis 213:584–591. doi: 10.1093/infdis/jiv323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peltola V, Waris M, Osterback R, Susi P, Ruuskanen O, Hyypia T. 2008. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J Infect Dis 197:382–389. doi: 10.1086/525542. [DOI] [PubMed] [Google Scholar]
- 12.van Benten I, Koopman L, Niesters B, Hop W, van Middelkoop B, de Waal L, van Drunen K, Osterhaus A, Neijens H, Fokkens W. 2003. Predominance of rhinovirus in the nose of symptomatic and asymptomatic infants. Pediatr Allergy Immunol 14:363–370. doi: 10.1034/j.1399-3038.2003.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zlateva KT, de Vries JJ, Coenjaerts FE, van Loon AM, Verheij T, Little P, Butler CC, Goossens H, Ieven M, Claas EC, GRACE Study Group. 2014. Prolonged shedding of rhinovirus and re-infection in adults with respiratory tract illness. Eur Respir J 44:169–177. doi: 10.1183/09031936.00172113. [DOI] [PubMed] [Google Scholar]
- 14.Gunnarsson RK, Holm SE, Soderstrom M. 1998. The prevalence of potential pathogenic bacteria in nasopharyngeal samples from healthy children and adults. Scand J Prim Health Care 16:13–17. [DOI] [PubMed] [Google Scholar]
- 15.da Gloria Carvalho M, Pimenta FC, Jackson D, Roundtree A, Ahmad Y, Millar EV, O’Brien KL, Whitney CG, Cohen AL, Beall BW. 2010. Revisiting pneumococcal carriage by use of broth enrichment and PCR techniques for enhanced detection of carriage and serotypes. J Clin Microbiol 48:1611–1618. doi: 10.1128/JCM.02243-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmu AA, Kaijalainen T, Saukkoriipi A, Leinonen M, Kilpi TM. 2012. Nasopharyngeal carriage of Streptococcus pneumoniae and pneumococcal urine antigen test in healthy elderly subjects. Scand J Infect Dis 44:433–438. doi: 10.3109/00365548.2011.652162. [DOI] [PubMed] [Google Scholar]
- 17.Regev-Yochay G, Raz M, Dagan R, Porat N, Shainberg B, Pinco E, Keller N, Rubinstein E. 2004. Nasopharyngeal carriage of Streptococcus pneumoniae by adults and children in community and family settings. Clin Infect Dis 38:632–639. doi: 10.1086/381547. [DOI] [PubMed] [Google Scholar]
- 18.van Deursen AM, van den Bergh MR, Sanders EA, Carriage Pilot Study Group. 2016. Carriage of Streptococcus pneumoniae in asymptomatic, community-dwelling elderly in the Netherlands. Vaccine 34:4–6. doi: 10.1016/j.vaccine.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Rawlings BA, Higgins TS, Han JK. 2013. Bacterial pathogens in the nasopharynx, nasal cavity, and osteomeatal complex during wellness and viral infection. Am J Rhinol Allergy 27:39–42. doi: 10.2500/ajra.2013.27.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett B, Locken K, Maberry R, Schwamman J, Brown R, Bobula J, Stauffacher EA. 2002. The Wisconsin Upper Respiratory Symptom Survey (WURSS): a new research instrument for assessing the common cold. J Fam Pract 51:265. [PubMed] [Google Scholar]
- 21.Barrett B, Brown R, Mundt M, Safdar N, Dye L, Maberry R, Alt J. 2005. The Wisconsin Upper Respiratory Symptom Survey is responsive, reliable, and valid. J Clin Epidemiol 58:609–617. doi: 10.1016/j.jclinepi.2004.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, Ortqvist A, Schaberg T, Torres A, van der Heijden G, Read R, Verheij TJ, Joint Taskforce of the European Respiratory Society and European Society for Clinical Microbiology and Infectious Diseases. 2011. Guidelines for the management of adult lower respiratory tract infections–full version. Clin Microbiol Infect 17:1–59. doi: 10.1111/j.1469-0691.2011.03602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamping DL, Schroter S, Marquis P, Marrel A, Duprat-Lomon I, Sagnier PP. 2002. The community-acquired pneumonia symptom questionnaire: a new, patient-based outcome measure to evaluate symptoms in patients with community-acquired pneumonia. Chest 122:920–929. doi: 10.1378/chest.122.3.920. [DOI] [PubMed] [Google Scholar]
- 24.Brittain-Long R, Nord S, Olofsson S, Westin J, Anderson LM, Lindh M. 2008. Multiplex real-time PCR for detection of respiratory tract infections. J Clin Virol 41:53–56. doi: 10.1016/j.jcv.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson ME, Olofsson S, Lindh M. 2014. Comparison of the FilmArray assay and in-house real-time PCR for detection of respiratory infection. Scand J Infect Dis 46:897–901. doi: 10.3109/00365548.2014.951681. [DOI] [PubMed] [Google Scholar]
- 26.Jartti T, Jartti L, Peltola V, Waris M, Ruuskanen O. 2008. Identification of respiratory viruses in asymptomatic subjects: asymptomatic respiratory viral infections. Pediatr Infect Dis J 27:1103–1107. doi: 10.1097/INF.0b013e31817e695d. [DOI] [PubMed] [Google Scholar]
- 27.Advani S, Sengupta A, Forman M, Valsamakis A, Milstone AM. 2012. Detecting respiratory viruses in asymptomatic children. Pediatr Infect Dis J 31:1221–1226. doi: 10.1097/INF.0b013e318265a804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singleton RJ, Bulkow LR, Miernyk K, DeByle C, Pruitt L, Hummel KB, Bruden D, Englund JA, Anderson LJ, Lucher L, Holman RC, Hennessy TW. 2010. Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol 82:1282–1290. doi: 10.1002/jmv.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Zalm MM, Uiterwaal CS, Wilbrink B, de Jong BM, Verheij TJ, Kimpen JL, van der Ent CK. 2009. Respiratory pathogens in respiratory tract illnesses during the first year of life: a birth cohort study. Pediatr Infect Dis J 28:472–476. doi: 10.1097/INF.0b013e318195e26e. [DOI] [PubMed] [Google Scholar]
- 30.van Gageldonk-Lafeber AB, Heijnen ML, Bartelds AI, Peters MF, van der Plas SM, Wilbrink B. 2005. A case-control study of acute respiratory tract infection in general practice patients in The Netherlands. Clin Infect Dis 41:490–497. doi: 10.1086/431982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jennings LC, Anderson TP, Beynon KA, Chua A, Laing RT, Werno AM, Young SA, Chambers ST, Murdoch DR. 2008. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax 63:42–48. doi: 10.1136/thx.2006.075077. [DOI] [PubMed] [Google Scholar]
- 32.Sundell N, Andersson LM, Brittain-Long R, Lindh M, Westin J. 2016. A four year seasonal survey of the relationship between outdoor climate and epidemiology of viral respiratory tract infections in a temperate climate. J Clin Virol 84:59–63. doi: 10.1016/j.jcv.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granados A, Goodall EC, Luinstra K, Smieja M, Mahony J. 2015. Comparison of asymptomatic and symptomatic rhinovirus infections in university students: incidence, species diversity, and viral load. Diagn Microbiol Infect Dis 82:292–296. doi: 10.1016/j.diagmicrobio.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O. 2004. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol 72:695–699. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]
- 35.Kling S, Donninger H, Williams Z, Vermeulen J, Weinberg E, Latiff K, Ghildyal R, Bardin P. 2005. Persistence of rhinovirus RNA after asthma exacerbation in children. Clin Exp Allergy 35:672–678. doi: 10.1111/j.1365-2222.2005.02244.x. [DOI] [PubMed] [Google Scholar]
- 36.Winther B, Hayden FG, Hendley JO. 2006. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: association with symptomatic illness and effect of season. J Med Virol 78:644–650. doi: 10.1002/jmv.20588. [DOI] [PubMed] [Google Scholar]
- 37.Graat JM, Schouten EG, Heijnen ML, Kok FJ, Pallast EG, de Greeff SC, Dorigo-Zetsma JW. 2003. A prospective, community-based study on virologic assessment among elderly people with and without symptoms of acute respiratory infection. J Clin Epidemiol 56:1218–1223. doi: 10.1016/S0895-4356(03)00171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fainstein V, Musher DM. 1979. Bacterial adherence to pharyngeal cells in smokers, nonsmokers, and chronic bronchitics. Infect Immun 26:178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipsky BA, Boyko EJ, Inui TS, Koepsell TD. 1986. Risk factors for acquiring pneumococcal infections. Arch Intern Med 146:2179–2185. doi: 10.1001/archinte.1986.00360230105016. [DOI] [PubMed] [Google Scholar]
- 40.Pastor P, Medley F, Murphy TV. 1998. Invasive pneumococcal disease in Dallas County, Texas: results from population-based surveillance in 1995. Clin Infect Dis 26:590–595. doi: 10.1086/514589. [DOI] [PubMed] [Google Scholar]
- 41.Raman AS, Swinburne AJ, Fedullo AJ. 1983. Pneumococcal adherence to the buccal epithelial cells of cigarette smokers. Chest 83:23–27. doi: 10.1378/chest.83.1.23. [DOI] [PubMed] [Google Scholar]