Abstract
Purpose
COPD is associated with coronary artery disease, and exacerbations are major events in COPD. However, the impact of recent hospitalized exacerbations on outcomes of percutaneous coronary intervention (PCI) remains underdetermined.
Patients and methods
Using the National Health Insurance Research Database of Taiwan, we identified 215,275 adult patients who underwent first-time PCI between 2000 and 2012. Among these patients, 15,485 patients had COPD. The risks of hospital mortality, overall mortality, and adverse cardiovascular outcomes after PCI (ie, ischemic events, repeat revascularization, cerebrovascular events, and major adverse cardiac and cerebrovascular events [MACCEs]) in relation to COPD, and the frequency and timing of recent hospitalized exacerbations within 1 year before PCI were estimated.
Results
COPD was independently associated with increased risks of hospital mortality, overall mortality, ischemic events, cerebrovascular events, and MACCE during follow-up after PCI. Among cerebrovascular events, ischemic rather than hemorrhagic stroke was more likely to occur. In COPD patients, recent hospitalized exacerbations further increased the risks of overall mortality, ischemic events, and MACCE following PCI. Notably, patients with more frequent or more recent hospitalized exacerbations had a trend toward higher risks of these adverse events (all P-values for trend <0.0001), especially those with ≥2 exacerbations within 1 year or any exacerbation within 1 month before PCI.
Conclusion
Integrated care is urgently needed to alleviate COPD-related morbidity and mortality after PCI, especially for patients with a recent hospitalized exacerbation.
Keywords: chronic obstructive pulmonary disease (COPD), exacerbation, percutaneous coronary intervention (PCI), cardiovascular outcomes
Introduction
COPD is highly prevalent and imposes an enormous and steadily increasing burden on affected individuals and health care systems.1 Over the past two decades, COPD has been a significant cause of worldwide mortality and morbidity.1,2 While respiratory failure is the primary cause of death in advanced COPD, cardiovascular disease, one of the leading causes of death in mild-to-moderate COPD, accounts for approximately one-third of mortalities in overall COPD patients.2,3
Evidence has suggested the link between COPD and the development of subclinical coronary atherosclerosis, coronary artery disease (CAD), and myocardial infarction.2–4 COPD exacerbations are major acute events and hospitalizations for an exacerbation usually occur in patients with severe disease.1,5,6 Risks of myocardial ischemia, as evidenced by cardiac troponin elevation, and even myocardial infarction may be increased following an exacerbation of COPD.3,7–10 These findings highlight the detrimental impact of COPD exacerbations on patients with concomitant CAD. The coexistence of COPD and CAD can be found in a substantial number of patients1,3,4,8 probably because of the high prevalence of both COPD and CAD and common inciting factors and pathogenesis, eg, smoking and systemic inflammation.4,11 The high prevalence of COPD and its potential risks in CAD patients provoked interest to explore whether COPD might worsen the prognosis of percutaneous coronary intervention (PCI),12–25 the most commonly used revascularization procedure. In these studies, early and late outcomes of COPD patients undergoing PCI have been documented. However, outcomes of patients experiencing recent hospitalized exacerbations before PCI have not been elucidated. Also, some studies demonstrated that COPD patients were at increased risk for hospital mortality,13,18,20 while others did not find an adverse effect of COPD.12,14–17,19 In the era of widespread use of PCI, the prognostic significance of COPD and recent hospitalized exacerbations need further clarification.
The National Health Insurance Research Database (NHIRD) of Taiwan provides valuable real-world big data that reflect different facets of respiratory diseases and cardiovascular procedures.26–29 Using the NHIRD of Taiwan, we sought to examine the hypothesis that COPD and recent hospitalized exacerbation might be associated with increased risks of hospital mortality and adverse outcomes during the follow-up period after PCI.
Materials and methods
Study cohort
This retrospective cohort study was conducted using the NHIRD of Taiwan, in which the ICD, Ninth Revision, Clinical Modification is used to define diseases and procedures.26–29 The accuracy of diagnoses in the NHIRD regarding respiratory (eg, COPD) and cardiovascular (eg, acute coronary syndrome) diseases has been previously validated.27,29 The codes used in this study are listed in Table S1. We accessed the de-identified data in the NHIRD under the approval of the Review Committee of the National Health Research Institutes. The whole study was approved by the Institutional Review Board of National Cheng Kung University Hospital (approval number: B-EX-106-017).
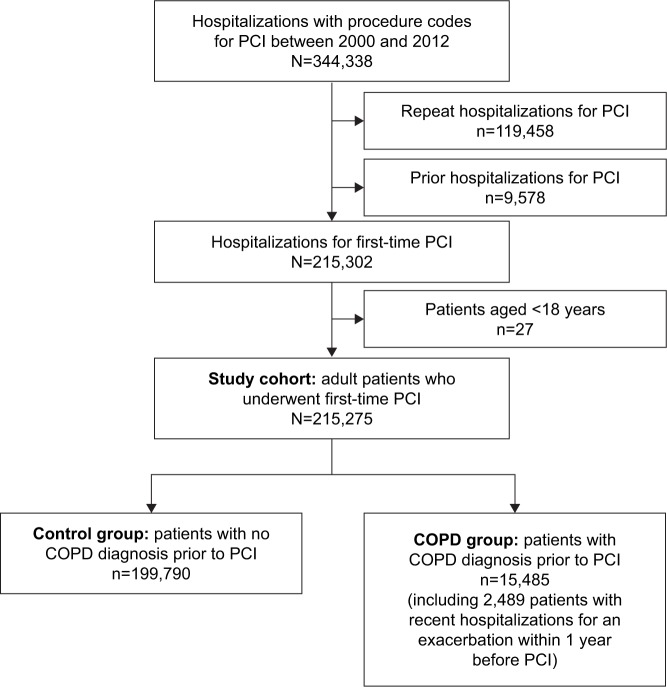
The flow diagram (Figure 1) shows the procedure of study cohort assembly. The inpatient hospital claims of the NHIRD provide the data of all the hospitalized patients undergoing PCI, including percutaneous transluminal coronary angioplasty and coronary stenting, from 1 January, 2000 to 31 December, 2012. During the study period, there were 344,338 hospitalizations for PCI. Among these hospitalizations, repeat hospitalizations for PCI, patients with prior hospitalizations for PCI from 1 January, 1996 to 31 December, 1999 and patients aged <18 years were excluded, ending up with 215,275 adult patients who underwent first-time PCI (ie, the index hospitalization) identified as the study cohort. In the PCI cohort, 15,485 (7.2%) patients who had a COPD diagnosis before PCI were considered to be the COPD group. Patients who did not have COPD were recognized as the control group. Among patients with COPD, 2,489 (16.1%) patients had been hospitalized for an exacerbation within 1 year before PCI. To assess the impact of recent hospitalized exacerbations, we categorized our study cohort based on the frequency (no exacerbation, one exacerbation, and ≥2 exacerbations within 1 year before PCI) and timing (no exacerbation within 1 year, any exacerbation during 6 months–1 year, any exacerbation during 1–6 months, and any exacerbation within 1 month before PCI). If the patient had experienced more than one hospitalized exacerbation within 1 year before PCI, the most recent episode was counted for analysis.
Figure 1.
Study flowchart summarizing cohort assembly of 215,275 adult patients undergoing first-time PCI between 2000 and 2012 in the NHIRD of Taiwan.
Abbreviations: NHIRD, National Health Insurance Research Database; PCI, percutaneous coronary intervention.
Comorbidities and outcome measurements
Baseline medical comorbidities, including hypertension, diabetes mellitus (DM), hyperlipidemia, congestive heart failure (CHF), chronic kidney disease, peripheral artery disease, prior stroke, dementia, and Parkinson’s disease, were identified according to the data of the index hospitalizations and previous hospitalizations as early as 1996. A patient with chronic kidney disease was considered to be end-stage renal disease when being registered with catastrophic illness certificates for dialysis. Also, whether the patients were admitted for acute coronary syndrome was identified. The accreditation levels of the hospitals (ie, medical centers, regional hospitals, or district hospitals) at which the patients underwent the PCI procedures were recorded.
The outcomes of hospital mortality, overall mortality, and adverse cardiovascular events during the follow-up period after PCI were analyzed. Ischemic events were defined as rehospitalizations for acute coronary syndrome. Repeat revascularization was defined as rehospitalizations for either coronary artery bypass grafting or re-PCI. Cerebrovascular events were defined based on rehospitalizations coded with ischemic stroke, hemorrhagic stroke, transient ischemic attack, subarachnoid hemorrhage, or other acute ill-defined cerebrovascular diseases. A composite end point, major adverse cardiac and cerebrovascular events (MACCEs), was defined as any occurrence of mortality, myocardial infarction, cerebrovascular events, and repeat revascularization.30 The entire cohort was evaluated for any occurrence of the selected adverse outcomes that occurred prior to censoring events, including mortality, withdrawal from the insurance program or December 31, 2013. For patients experiencing more than one episode of each outcome, only the first episode was counted.
Statistical analysis
The COPD group was compared with the control group concerning baseline characteristics, procedural data, and outcomes. The chi-squared test or Fisher’s exact test was used to compare categorical variables, whereas the Student’s t-test was used to compare continuous variables as necessary. The OR of hospital mortality associated with COPD was estimated using logistic regression models with adjustment for potential confounding variables considered in relevant cardiovascular studies,26,31 including patient characteristics (ie, age, sex, and baseline medical comorbidities), acute coronary syndrome, stenting, and hospital levels. The HRs of overall mortality and adverse cardiovascular outcomes associated with COPD were estimated using Cox proportional hazards regression models with adjustment for potential confounding variables. Kaplan–Meier curves were generated to evaluate overall survival and freedom from myocardial infarction, repeat revascularization, cerebrovascular events, and MACCE after PCI. The OR of hospital mortality and HRs of overall mortality and adverse cardiovascular outcomes associated with the frequency or timing of recent hospitalized exacerbations were estimated. Analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC, USA) and Stata/SE 14.0 for Windows (StataCorp, College Station, TX, USA). All statistical significances were set at a P<0.05.
Results
Baseline characteristics and procedures
Table 1 summarizes the baseline characteristics and procedural data between patients with and without COPD in the PCI cohort. Of the 199,790 patients who did not have COPD, the mean age was 64.7 years. The male-to-female ratio was 2.56. The mean total hospital costs were NTD159,940, equivalent to approximately USD5,382 or €4,363.
Table 1.
Comparison of patient characteristics between patients without COPD (control group) and patients with COPD in the PCI cohort of Taiwan, 2000–2012
| Characteristicsa | Controls (n=199,790) | COPD (n=15,485) | P-value |
|---|---|---|---|
| Age group | |||
| <50 years old | 25,032 (12.5) | 239 (1.5) | <0.0001 |
| 50–59 years old | 46,144 (23.1) | 1,176 (7.6) | |
| 60–69 years old | 54,402 (27.2) | 3,099 (20.0) | |
| 70–79 years old | 53,501 (26.8) | 6,466 (41.8) | |
| ≥80 years old | 20,711 (10.4) | 4,505 (29.1) | |
| Age, mean (SD) | 64.7 (12.2) | 74.0 (9.6) | <0.0001 |
| Sexb | |||
| Female | 56,152 (28.1) | 3,824 (24.7) | <0.0001 |
| Male | 143,531 (71.9) | 11,661 (75.3) | |
| Medical comorbidities | |||
| Hypertension | 131,344 (65.7) | 12,455 (80.4) | <0.0001 |
| DM | 77,810 (39.0) | 7,083 (45.7) | <0.0001 |
| Hyperlipidemia | 82,102 (41.1) | 5,307 (34.3) | <0.0001 |
| CHF | 32,753 (16.4) | 6,440 (41.6) | <0.0001 |
| Chronic kidney disease | 10,758 (5.4) | 1,643 (10.6) | <0.0001 |
| End-stage renal disease | 8,959 (4.5) | 981 (6.4) | <0.0001 |
| Peripheral artery disease | 5,162 (2.6) | 908 (5.9) | <0.0001 |
| Prior stroke | 26,881 (13.5) | 4,723 (30.5) | <0.0001 |
| Dementia | 1,814 (0.9) | 531 (3.4) | <0.0001 |
| Parkinson’s disease | 1,621 (0.8) | 378 (2.4) | <0.0001 |
| Acute coronary syndrome | 113,686 (56.9) | 8,003 (51.7) | <0.0001 |
| Stenting | 97,324 (48.7) | 5,410 (34.9) | <0.0001 |
| Hospital level | |||
| Medical center | 81,665 (40.9) | 5,378 (34.7) | <0.0001 |
| Regional hospital | 93,905 (47.0) | 7,902 (51.0) | |
| District hospital | 24,220 (12.1) | 2,205 (14.2) | |
| Total hospital costs (NTD, mean ± SD) | 159,940±101,232 | 175,392±117,928 | <0.0001 |
Notes:
Data are expressed as patient number (percentage) unless otherwise indicated.
Gender data were missing or undetermined in 107 (0.05%) patients.
Abbreviations: CHF, congestive heart failure; DM, diabetes mellitus; NTD, New Taiwan Dollar; PCI, percutaneous coronary intervention.
Compared with the control group, COPD patients were significantly older, with an even higher male-to-female ratio of 3.05. COPD patients frequently had multiple comorbidities. These patients were less likely to undergo PCI at the occurrence of acute coronary syndrome as compared to the control group and were also less likely to receive coronary stenting during PCI. For COPD patients, the PCI procedures were less likely to be conducted in medical centers, but the hospital costs were significantly higher than that of the control group.
Mortality and adverse cardiovascular outcomes
The OR of hospital mortality and HRs of overall mortality and adverse cardiovascular outcomes associated with COPD are summarized in Table 2. Following PCI, COPD was independently associated with an increased risk of hospital mortality after adjustment for potential confounding variables (adjusted OR: 1.13, 95% CI: 1.03–1.23, P=0.01). Also, COPD was independently associated with overall mortality, ischemic events, myocardial infarction, and cerebrovascular events. Although COPD was not associated with repeat revascularization, it appeared to mildly increase the risk of repeat PCI. Overall, COPD patients had a higher risk of MACCE after PCI. Among subtypes of cerebrovascular events (Table 3), ischemic stroke and transient ischemic attack, but not hemorrhagic stroke and subarachnoid hemorrhage, were more likely to occur in COPD patients.
Table 2.
OR of hospital mortality and HRs of overall mortality and adverse cardiovascular outcomes associated with COPD in the PCI cohort
| Outcomes | Controls (n=199,790) | COPD (n=15,485) |
|---|---|---|
| Hospital mortality (%) | 4,442 (2.2) | 618 (4.0) |
| Crude OR (95% CI) | 1.00 | 1.83 (1.68–1.99)*** |
| Adjusted OR (95% CI)a | 1.00 | 1.13 (1.03–1.23)* |
| Overall mortality (%) | 55,744 (27.9) | 8,880 (57.4) |
| Crude HR (95% CI) | 1.00 | 2.72 (2.66–2.78)**** |
| Adjusted HR (95% CI)b | 1.00 | 1.35 (1.32–1.38)**** |
| Ischemic events (%) | 59,259 (29.7) | 5,260 (34.0) |
| Crude HR (95% CI) | 1.00 | 1.49 (1.44–1.53)**** |
| Adjusted HR (95% CI)b | 1.00 | 1.33 (1.29–1.37)**** |
| Myocardial infarction (%) | 22,086 (11.1) | 2,072 (12.4) |
| Crude HR (95% CI) | 1.00 | 1.52 (1.46–1.59)**** |
| Adjusted HR (95% CI)b | 1.00 | 1.22 (1.16–1.28)**** |
| Repeat revascularization (%) | 76,189 (38.1) | 5,019 (32.4) |
| Crude HR (95% CI) | 1.00 | 1.00 (0.97–1.03) |
| Adjusted HR (95% CI)b | 1.00 | 1.01 (0.98–1.04) |
| Repeat PCI (%) | 72,218 (36.2) | 4,804 (31.0) |
| Crude HR (95% CI) | 1.00 | 1.01 (0.98–1.04) |
| Adjusted HR (95% CI)b | 1.00 | 1.03 (1.00–1.07)* |
| Cerebrovascular events (%) | 24,165 (12.1) | 2,585 (16.7) |
| Crude HR (95% CI) | 1.00 | 1.93 (1.85–2.01)**** |
| Adjusted HR (95% CI)b | 1.00 | 1.12 (1.08–1.17)**** |
| MACCE (%) | 120,531 (60.3) | 11,877 (76.7) |
| Crude HR (95% CI) | 1.00 | 1.87 (1.84–1.91)**** |
| Adjusted HR (95% CI)b | 1.00 | 1.25 (1.22–1.27)**** |
Notes:
Based on the multiple logistic regression analysis with adjustment for age, sex, medical comorbidities, acute coronary syndrome, stenting, and hospital levels.
Based on the Cox proportional hazards regression model with adjustment for age, sex, medical comorbidities, acute coronary syndrome, stenting, and hospital levels.
P<0.05,
P<0.001,
P<0.0001.
Abbreviations: MACCE, major adverse cardiac and cerebrovascular event; PCI, percutaneous coronary intervention.
Table 3.
Adjusted HRs of cerebrovascular events and subtypes associated with COPD in the PCI cohort
| Outcomes | Controls (n=199,790) | COPD (n=15,485) |
|---|---|---|
| Ischemic stroke | 18,351 (9.2) | 1,901 (12.3) |
| Adjusted HR (95% CI)a | 1.00 | 1.07 (1.02–1.13)** |
| Hemorrhagic stroke | 3,094 (1.6) | 254 (1.6) |
| Adjusted HR (95% CI)a | 1.00 | 0.88 (0.77–1.01) |
| Transient ischemic attack | 5,013 (2.5) | 662 (4.3) |
| Adjusted HR (95% CI)a | 1.00 | 1.38 (1.26–1.50)**** |
| Subarachnoid hemorrhage | 471 (0.2) | 34 (0.2) |
| Adjusted HR (95% CI)a | 1.00 | 0.91 (0.64–1.30) |
Notes:
Based on the Cox proportional hazards regression model with adjustment for age, sex, medical comorbidities, acute coronary syndrome, stenting, and hospital levels.
P<0.01,
P<0.0001.
Abbreviation: PCI, percutaneous coronary intervention.
The impact of recent hospitalized exacerbations on the outcomes
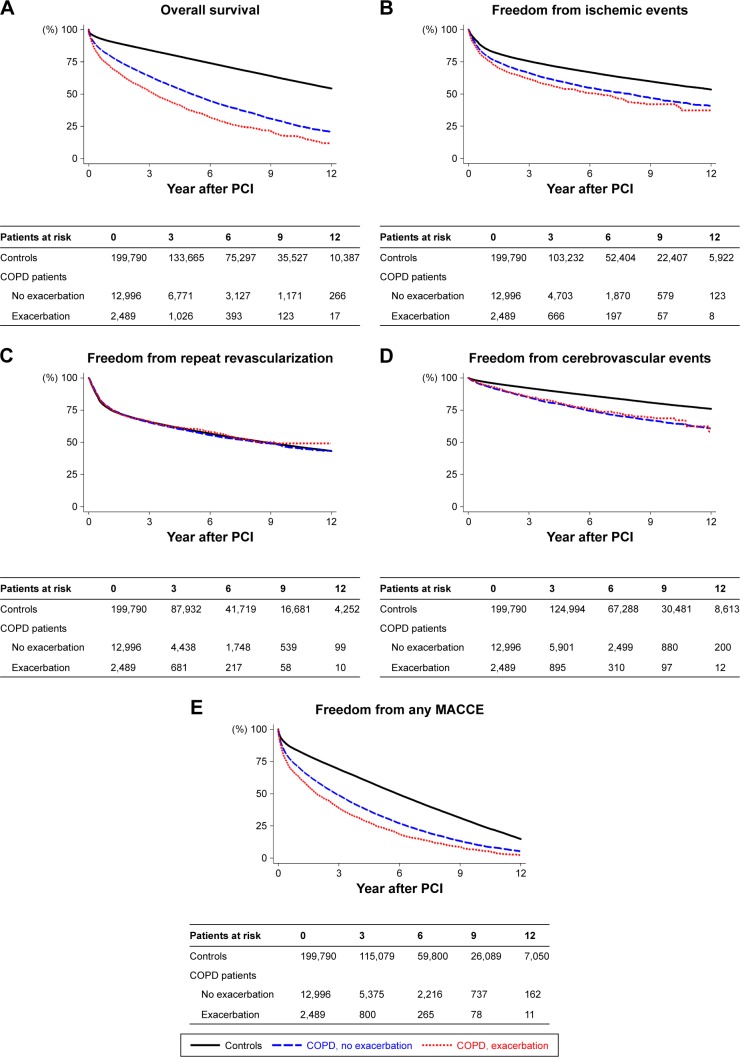
Figure 2A–E shows the Kaplan–Meier estimates of overall survival and freedom from ischemic events, repeat revascularization, cerebrovascular events, and any MACCE during follow-up after PCI in patients without COPD, COPD patients without recent hospitalized exacerbations, and those with any exacerbation within 1 year before PCI. The OR of hospital mortality and HRs of overall mortality and adverse cardiovascular outcomes associated with COPD and the frequency of recent hospitalized exacerbations within 1 year before PCI are presented in Table 4. Compared with the control group, the risks of hospital mortality and cerebrovascular events following PCI were increased in COPD patients, but not significant in those who had recent hospitalized exacerbations. Also, COPD patients had significantly higher risks of overall mortality, ischemic events, and MACCE following PCI than the control group, regardless of the recent hospitalized exacerbation and its frequency. In COPD patients, the recent hospitalized exacerbations further increased the risks of overall mortality, ischemic events, and MACCE regardless of the frequency. Notably, patients with more frequent hospitalized exacerbations corresponded to higher risks of overall mortality, ischemic events, and MACCE (all P-values for trend <0.0001). The OR of hospital mortality and HRs of overall mortality and adverse cardiovascular outcomes associated with COPD and the timing of recent hospitalized exacerbations within 1 year before PCI are presented in Table 5. Similarly, COPD patients had significantly higher risks of overall mortality, ischemic events, and MACCE following PCI than the control group regardless of the recent hospitalized exacerbation and its timing. COPD only increased the risk of repeat revascularization when any exacerbation occurred within 1 month before PCI. In COPD patients, the recent hospitalized exacerbations further increased the risk of overall mortality regardless of the timing. The risk of MACCE was further increased when any exacerbation occurred within 6 months and the risks of ischemic events and repeat revascularization were increased when any exacerbation within 1 month before PCI. Notably, more recent hospitalized exacerbations corresponded to higher risks of overall mortality, ischemic events, and MACCE (all P-values for trend <0.0001).
Figure 2.
Kaplan–Meier estimates of (A) overall survival, (B) freedom from ischemic events, (C) freedom from repeat revascularization, (D) freedom from cerebrovascular events, and (E) freedom from any MACCE in patients without COPD (controls), COPD patients with no recent exacerbation, and patients with any exacerbation within 1 year before PCI.
Abbreviations: MACCE, major adverse cardiac and cerebrovascular event; PCI, percutaneous coronary intervention.
Table 4.
Adjusted OR of hospital mortality and HRs of overall mortality and adverse cardiovascular outcomes association with COPD and the frequency of recent hospitalized exacerbations within 1 year before PCI
| Outcomes | Frequency of recent hospitalized exacerbations within 1 year before PCI in patients of COPD | ||
|---|---|---|---|
| No exacerbation (n=12,996) | One exacerbation (n=1,909) | ≥2 exacerbations (n=580) | |
| Reference: controls (n=199,790) | |||
| Hospital mortalitya | 1.12 (1.01–1.23)* | 1.19 (0.95–1.50) | 1.10 (0.72–1.67) |
| Overall mortalityb | 1.31 (1.28–1.34)**** | 1.57 (1.48–1.66)**** | 1.72 (1.55–1.90)**** |
| Ischemic eventsb | 1.30 (1.26–1.35)**** | 1.45 (1.34–1.57)**** | 1.63 (1.42–1.88)**** |
| Repeat revascularizationb | 1.02 (0.99–1.05) | 1.02 (0.94–1.10) | 0.88 (0.75–1.04) |
| Cerebrovascular eventsb | 1.14 (1.09–1.19)**** | 1.06 (0.94–1.19) | 0.93 (0.73–1.18) |
| MACCEb | 1.22 (1.19–1.25)**** | 1.39 (1.32–1.47)**** | 1.52 (1.38–1.66)**** |
| Reference: COPD, no exacerbation (n=12,996) | |||
| Hospital mortalitya | – | 1.07 (0.84–1.36) | 0.98 (0.64–1.51) |
| Overall mortalityb | – | 1.20 (1.13–1.27)**** | 1.31 (1.19–1.45)**** |
| Ischemic eventsb | – | 1.11 (1.03–1.21)* | 1.25 (1.09–1.45)** |
| Repeat revascularizationb | – | 1.00 (0.92–1.09) | 0.87 (0.87–1.02) |
| Cerebrovascular eventsb | – | 0.93 (0.82–1.05) | 0.82 (0.64–1.04) |
| MACCEb | – | 1.14 (1.08–1.21)**** | 1.24 (1.13–1.36)**** |
Notes:
Adjusted OR estimated based on the multiple logistic regression analysis with adjustment for age, sex, medical comorbidities, acute coronary syndrome, stenting, and hospital levels.
Adjusted HR estimated based on the Cox proportional hazards regression model with adjustment for age, sex, medical comorbidities, acute coronary syndrome, stenting, and hospital levels.
P<0.05,
P<0.01,
P<0.0001.
Abbreviations: MACCE, major adverse cardiac and cerebrovascular event; PCI, percutaneous coronary intervention.
Table 5.
Adjusted OR of hospital mortality and HRs of overall mortality and adverse cardiovascular outcomes association with COPD and the timing of recent hospitalized exacerbations within 1 year before PCI
| Outcomes | Timing of recent hospitalized exacerbations within 1 year before PCI in patients with COPD | |||
|---|---|---|---|---|
| No exacerbation within 1 year (n=12,996) | Exacerbation at 6 months–1 year (n=688) | Exacerbation at 1–6 months (n=1,031) | Exacerbation within 1 month (n=790) | |
| Reference: controls (n=199,790) | ||||
| Hospital mortalitya | 1.12 (1.01–1.23)* | 1.19 (0.82–1.73) | 1.08 (0.78–1.50) | 1.27 (0.90–1.79) |
| Overall mortalityb | 1.31 (1.28–1.34)**** | 1.56 (1.42–1.71)**** | 1.60 (1.48–1.73)**** | 1.62 (1.48–1.76)**** |
| Ischemic eventsb | 1.30 (1.26–1.35)**** | 1.41 (1.24–1.61)**** | 1.45 (1.30–1.61)**** | 1.60 (1.42–1.80)**** |
| Repeat revascularizationb | 1.02 (0.99–1.05) | 0.89 (0.77–1.03) | 0.92 (0.82–1.03) | 1.16 (1.02–1.31)* |
| Cerebrovascular eventsb | 1.14 (1.09–1.19)**** | 0.97 (0.79–1.19) | 1.10 (0.94–1.29) | 0.95 (0.77–1.16) |
| MACCEb | 1.22 (1.19–1.25)**** | 1.30 (1.19–1.41)**** | 1.43 (1.33–1.53)**** | 1.50 (1.39–1.62)**** |
| Reference: COPD, no exacerbation (n=12,996) | ||||
| Hospital mortalitya | – | 1.06 (0.72–1.57) | 0.97 (0.69–1.35) | 1.14 (0.80–1.62) |
| Overall mortalityb | – | 1.19 (1.08–1.31)*** | 1.22 (1.13–1.32)**** | 1.24 (1.13–1.35)**** |
| Ischemic eventsb | – | 1.08 (0.95–1.24) | 1.11 (1.00–1.24) | 1.23 (1.09–1.39)** |
| Repeat revascularizationb | – | 0.88 (0.76–1.01) | 0.90 (0.80–1.02) | 1.14 (1.00–1.29)* |
| Cerebrovascular eventsb | – | 0.85 (0.69–1.05) | 0.97 (0.82–1.14) | 0.83 (0.68–1.02) |
| MACCEb | – | 1.06 (0.97–1.16) | 1.17 (1.09–1.26)**** | 1.23 (1.13–1.33)**** |
Notes:
Adjusted OR estimated based on the multiple logistic regression analysis with adjustment for age, sex, medical comorbidities, acute coronary syndrome, stenting, and hospital levels.
Adjusted HR estimated based on the Cox proportional hazards regression model with adjustment for age, sex, medical comorbidities, acute coronary syndrome, stenting, and hospital levels.
P<0.05,
P<0.01,
P<0.001,
P<0.0001.
Abbreviations: MACCE, major adverse cardiac and cerebrovascular event; PCI, percutaneous coronary intervention.
Discussion
Some studies have been performed to explore the impact of COPD on the outcomes of PCI in depth using hospital or registry databases, yet the early outcome regarding COPD patients vs those without COPD remained inconsistent.12–20 As found in our study, COPD patients were commonly older and had multiple comorbidities, which may increase the risk of hospital mortality. Notably, even after controlling for potential confounding factors, COPD increased a modest but significant risk of hospital mortality following PCI. Such a modest increase in the risk of hospital mortality may explain the inconsistent results reported in the literature.12–20
One of most significant findings of this study is to demonstrate the clinical significance of recent hospitalized exacerbations in PCI outcomes. In accordance with previous studies,12–15,18,21–25 we found that COPD itself was associated with increased risks of overall mortality and adverse cardiovascular outcomes after PCI. It has been known that hospitalization for an exacerbation is associated with an increased risk of death in COPD.1,5 In this study, we did not find recent hospitalized exacerbations before PCI significantly associated with increased hospital mortality. The potential explanation is that physicians may be adept at selecting candidates for PCI based on the highest probability of survival and provide more aggressive treatment for these patients. However, we found that recent hospitalized exacerbations further increased the risk of overall mortality during followup. Patients with more frequent or more recent hospitalized exacerbations before PCI were both at higher risk of overall mortality. An exacerbation of COPD can trigger systemic inflammation that leads to an increased risk of myocardial damage.7,9 The relationship between COPD exacerbations and the subsequent risk of cardiovascular events has been established.9,10 The risk remains elevated up to 1 year after exacerbation.10 Consistently, we demonstrated that patients with more frequent or more recent hospitalized exacerbations within 1 year before PCI corresponded to higher risks of adverse cardiovascular events during follow-up, especially those with ≥2 exacerbations within 1 year or any exacerbation within 1 month before PCI. Our findings suggest that the detrimental effect of recent hospitalized exacerbations might not be fully offset by coronary revascularization using PCI. Organizing integrated care is required to improve clinical outcomes of this high-risk population.
To date, only a few studies have attempted to investigate whether COPD is associated with cerebrovascular events after PCI and none reported a positive association.14,22,23 On the contrary, the present study provided evidence with a large patient number showing that COPD may contribute to cerebrovascular events after PCI. Recently, a prospective population-based study demonstrated increased risks of ischemic and hemorrhagic stroke in COPD patients.32 However, we found more ischemic stroke events rather than hemorrhagic stroke occurring in patients with COPD undergoing PCI. The potential explanation might be that antiplatelet therapy, an evidence-based strategy to alleviate the threats of ischemic stroke and transient ischemic attack,33 was less likely to be prescribed in COPD patients undergoing PCI.15,25 Whether these patients might benefit from the strategies preventing neurologic complications warrants further investigation.
Our study had some limitations. First, COPD in this study was leniently defined by clinical diagnoses rather than spirometry which is considered as gold standard, though the accuracy of COPD diagnosis in the NHIRD has been validated.27 Probably because of the major concern regarding the use of spirometry in patients presenting with heart attack,8,34 spirometry was systemically applied to determine all participants with COPD in only one among related PCI studies.24 Our approach was used in previous studies,13,18,19,22,23,25 and has been considered robust enough to identify patients with real-world COPD.15 In addition, a fraction of data missing or miscoding is inherent in studies using a large database, eg, COPD exacerbation accompanied by cardiac events or cardiac events per se being potentially misclassified as COPD exacerbation because of their similar clinical presentation, but such errors are likely random from a nationwide database and should not be a valid argument for our findings as nondifferential misclassification may lead to a bias toward the null. Second, some variables of interest, eg, body mass index, lipid levels, HBA1C, the severity of CAD, the status of smoking, echocardiographic findings, and completeness of revascularization and medications, were unavailable in the database and thus cannot be analyzed. Alternatively, hyperlipidemia, DM, and CHF have been considered in the models when multiple logistic regression or Cox proportional hazards regression analysis was performed. Finally, massive reimbursement cuts of cardiac care, as evidenced by low hospital costs, might be harmful to the quality of care.35 This restricted medical expenditure could bring more detrimental impact on COPD patients who underwent PCI, as multidisciplinary care is inevitably mandatory for these patients. Validation of our results in other health care systems may be necessary.
Conclusion
Our study further demonstrates that patients with recent hospitalized exacerbations within 1 year before PCI had higher risks of adverse outcomes after PCI. Integrated care is urgently needed to alleviate COPD-related morbidity and mortality after PCI, especially for patients with a recent hospitalized exacerbation.
Supplementary material
Table S1.
ICD, Ninth Revision, Clinical Modification (ICD-9-CM) codes of procedures, comorbidities, and adverse outcomes
| Variables | ICD-9-CM code |
|---|---|
| Procedure | |
| Percutaneous coronary intervention | 00.66, 36.01, 36.02, 36.05, 36.06, 36.07, 36.09 |
| Coronary artery stenting | 36.06, 36.07 |
| Comorbidity | |
| Chronic obstructive pulmonary disease | 490–492, 496 |
| Acute exacerbation | 491.21 |
| Hypertension | 401–405, 437.2 |
| DM | 250, 357.2, 362.0, 366.41 |
| Hyperlipidemia | 272.0–272.4 |
| CHF | 428 |
| Chronic kidney disease | 585 |
| Peripheral artery disease | 443, 444 |
| Prior stroke | 430–438 |
| Dementia | 290, 294.1, 294.8, 331.0, 331.1, 331.8 |
| Parkinson’s disease | 332 |
| Acute coronary syndrome | 410, 411, 413 |
| Adverse outcome | |
| Coronary artery bypass grafting | 36.1 |
| Cerebrovascular events | 430–436 |
| Ischemic stroke (artery occlusion) | 433, 434 |
| Hemorrhagic stroke | 431, 432 |
| Transient ischemic attack | 435 |
| Subarachnoid hemorrhage | 430 |
| Acute myocardial infarction | 410 |
Abbreviations: CHF, congestive heart failure; DM, diabetes mellitus.
Acknowledgments
The authors are grateful to Sheau-Chiann Chen, PhD, for providing the statistical consulting services from the Center for Quantitative Sciences, Vanderbilt University Medical Center, Nashville, USA. This work was supported by National Cheng Kung University Hospital, Tainan, Taiwan (NCKUH-10503025 to CHL and NCKUH-10706007 to WCL).
Footnotes
Author contributions
CYL and CHL were the guarantors of this paper. WCL, CWC, WWL, MHH, LMT, CYL, and CHL helped in the study concept and design. All authors performed the acquisition and interpretation of data. WCL, CYL, and CHL helped in drafting of the manuscript. CLL, CYL, and CHL performed the statistical analysis. CYL and CHL were involved in the study supervision. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 2.Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28(6):1245–1257. doi: 10.1183/09031936.00133805. [DOI] [PubMed] [Google Scholar]
- 3.Rothnie KJ, Yan R, Smeeth L, Quint JK. Risk of myocardial infarction (MI) and death following MI in people with chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMJ Open. 2015;5(9):e007824. doi: 10.1136/bmjopen-2015-007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roversi S, Fabbri LM, Sin DD, Hawkins NM, Agustí A. Chronic obstructive pulmonary disease and cardiac diseases. An urgent need for integrated care. Am J Respir Crit Care Med. 2016;194(11):1319–1336. doi: 10.1164/rccm.201604-0690SO. [DOI] [PubMed] [Google Scholar]
- 5.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almagro P, Salvadó M, Garcia-Vidal C, et al. Recent improvement in long-term survival after a COPD hospitalisation. Thorax. 2010;65(4):298–302. doi: 10.1136/thx.2009.124818. [DOI] [PubMed] [Google Scholar]
- 7.Høiseth AD, Neukamm A, Karlsson BD, Omland T, Brekke PH, Søyseth V. Elevated high-sensitivity cardiac troponin T is associated with increased mortality after acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2011;66(9):775–781. doi: 10.1136/thx.2010.153122. [DOI] [PubMed] [Google Scholar]
- 8.Campo G, Pavasini R, Malagù M, et al. Chronic obstructive pulmonary disease and ischemic heart disease comorbidity: overview of mechanisms and clinical management. Cardiovasc Drugs Ther. 2015;29(2):147–157. doi: 10.1007/s10557-014-6569-y. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest. 2010;137(5):1091–1097. doi: 10.1378/chest.09-2029. [DOI] [PubMed] [Google Scholar]
- 10.Kunisaki KM, Dransfield MT, Anderson JA. Exacerbations of chronic obstructive pulmonary disease and cardiac events: a cohort analysis. Am J Respir Crit Care Med. 2018;198(1):51–57. doi: 10.1164/rccm.201711-2239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magnussen H, Watz H. Systemic inflammation in chronic obstructive pulmonary disease and asthma: relation with comorbidities. Proc Am Thorac Soc. 2009;6(8):648–651. doi: 10.1513/pats.200906-053DP. [DOI] [PubMed] [Google Scholar]
- 12.Berger JS, Sanborn TA, Sherman W, Brown DL. Effect of chronic obstructive pulmonary disease on survival of patients with coronary heart disease having percutaneous coronary intervention. Am J Cardiol. 2004;94(5):649–651. doi: 10.1016/j.amjcard.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 13.Selvaraj CL, Gurm HS, Gupta R, Ellis SG, Bhatt DL. Chronic obstructive pulmonary disease as a predictor of mortality in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2005;96(6):756–759. doi: 10.1016/j.amjcard.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Nishiyama K, Morimoto T, Furukawa Y, et al. Chronic obstructive pulmonary disease – an independent risk factor for long-term cardiac and cardiovascular mortality in patients with ischemic heart disease. Int J Cardiol. 2010;143(2):178–183. doi: 10.1016/j.ijcard.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Enriquez JR, Parikh SV, Selzer F, et al. Increased adverse events after percutaneous coronary intervention in patients with COPD: insights from the National Heart, Lung, and Blood Institute dynamic registry. Chest. 2011;140(3):604–610. doi: 10.1378/chest.10-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sung PH, Chung SY, Sun CK, et al. Impact of chronic obstructive pulmonary disease on patient with acute myocardial infarction undergoing primary percutaneous coronary intervention. Biomed J. 2013;36(6):274–281. doi: 10.4103/2319-4170.113373. [DOI] [PubMed] [Google Scholar]
- 17.Zhang JW, Zhou YJ, Yang Q, Yang SW, Nie B, Xu XH. Impact of chronic obstructive pulmonary diseases on outcomes and hospital days after percutaneous coronary intervention. Angiology. 2013;64(6):430–434. doi: 10.1177/0003319712458145. [DOI] [PubMed] [Google Scholar]
- 18.Jatene T, Biering-Sørensen T, Nochioka K, et al. Frequency of cardiac death and stent thrombosis in patients with chronic obstructive pulmonary disease undergoing percutaneous coronary intervention (from the BASKET-PROVE I and II Trials) Am J Cardiol. 2017;119(1):14–19. doi: 10.1016/j.amjcard.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Januszek R, Siudak Z, Dziewierz A, Rakowski T, Dudek D, Bartuś S. Chronic obstructive pulmonary disease affects the angiographic presentation and outcomes of patients with coronary artery disease treated with percutaneous coronary interventions. Pol Arch Intern Med. 2018;128(1):24–34. doi: 10.20452/pamw.4145. [DOI] [PubMed] [Google Scholar]
- 20.Șerban RC, Hadadi L, Șuș I, Lakatos EK, Demjen Z, Scridon A. Impact of chronic obstructive pulmonary disease on in-hospital morbidity and mortality in patients with ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention. Int J Cardiol. 2017;243:437–442. doi: 10.1016/j.ijcard.2017.05.044. [DOI] [PubMed] [Google Scholar]
- 21.Konecny T, Somers K, Orban M, et al. Interactions between COPD and outcomes after percutaneous coronary intervention. Chest. 2010;138(3):621–627. doi: 10.1378/chest.10-0300. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Cheng YJ, Zheng WP, et al. Impact of chronic obstructive pulmonary disease on long-term outcome in patients with coronary artery disease undergoing percutaneous coronary intervention. Biomed Res Int. 2016;2016:1–6. doi: 10.1155/2016/8212459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campo G, Guastaroba P, Marzocchi A, et al. Impact of COPD on long-term outcome after ST-segment elevation myocardial infarction receiving primary percutaneous coronary intervention. Chest. 2013;144(3):750–757. doi: 10.1378/chest.12-2313. [DOI] [PubMed] [Google Scholar]
- 24.Almagro P, Lapuente A, Pareja J, et al. Underdiagnosis and prognosis of chronic obstructive pulmonary disease after percutaneous coronary intervention: a prospective study. Int J Chron Obstruct Pulmon Dis. 2015;10:1353–1361. doi: 10.2147/COPD.S84482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andell P, Sjögren J, Batra G, Szummer K, Koul S. Outcome of patients with chronic obstructive pulmonary disease and severe coronary artery disease who had a coronary artery bypass graft or a percutaneous coronary intervention. Eur J Cardiothorac Surg. 2017;52(5):930–936. doi: 10.1093/ejcts/ezx219. [DOI] [PubMed] [Google Scholar]
- 26.Lai CH, Lai WW, Chiou MJ, et al. Outcomes of percutaneous coronary intervention in patients with rheumatoid arthritis and systemic lupus erythematosus: an 11-year nationwide cohort study. Ann Rheum Dis. 2016;75(7):1350–1356. doi: 10.1136/annrheumdis-2015-207719. [DOI] [PubMed] [Google Scholar]
- 27.Su VY, Yang KY, Yang YH, et al. Use of ICS/LABA combinations or LAMA is associated with a lower risk of acute exacerbation in patients with coexistent COPD and asthma. J Allergy Clin Immunol Pract. 2018;6(6):1927–1935. doi: 10.1016/j.jaip.2018.01.035. [DOI] [PubMed] [Google Scholar]
- 28.Lai CH, Lai WW, Chiou MJ, Tsai LM, Wen JS, Li CY. Outcomes of coronary artery bypass grafting in patients with inflammatory rheumatic diseases: an 11-year nationwide cohort study. J Thorac Cardiovasc Surg. 2015;149(3):859–866. doi: 10.1016/j.jtcvs.2014.11.038. [DOI] [PubMed] [Google Scholar]
- 29.Cheng CL, Lee CH, Chen PS, Li YH, Lin SJ, Yang YH. Validation of acute myocardial infarction cases in the national health insurance research database in Taiwan. J Epidemiol. 2014;24(6):500–507. doi: 10.2188/jea.JE20140076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kip KE, Hollabaugh K, Marroquin OC, Williams DO. The problem with composite end points in cardiovascular studies: the story of major adverse cardiac events and percutaneous coronary intervention. J Am Coll Cardiol. 2008;51(7):701–707. doi: 10.1016/j.jacc.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 31.Hueb W, Lopes N, Gersh BJ, et al. Ten-year follow-up survival of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation. 2010;122(10):949–957. doi: 10.1161/CIRCULATIONAHA.109.911669. [DOI] [PubMed] [Google Scholar]
- 32.Portegies ML, Lahousse L, Joos GF, et al. Chronic obstructive pulmonary disease and the risk of stroke. The Rotterdam study. Am J Respir Crit Care Med. 2016;193(3):251–258. doi: 10.1164/rccm.201505-0962OC. [DOI] [PubMed] [Google Scholar]
- 33.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 34.Cooper BG. An update on contraindications for lung function testing. Thorax. 2011;66(8):714–723. doi: 10.1136/thx.2010.139881. [DOI] [PubMed] [Google Scholar]
- 35.Chang GM, Cheng SH, Tung YC. Impact of cuts in reimbursement on outcome of acute myocardial infarction and use of percutaneous coronary intervention: a nationwide population-based study over the period 1997 to 2008. Med Care. 2011;49(12):1054–1061. doi: 10.1097/MLR.0b013e318235382b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
ICD, Ninth Revision, Clinical Modification (ICD-9-CM) codes of procedures, comorbidities, and adverse outcomes
| Variables | ICD-9-CM code |
|---|---|
| Procedure | |
| Percutaneous coronary intervention | 00.66, 36.01, 36.02, 36.05, 36.06, 36.07, 36.09 |
| Coronary artery stenting | 36.06, 36.07 |
| Comorbidity | |
| Chronic obstructive pulmonary disease | 490–492, 496 |
| Acute exacerbation | 491.21 |
| Hypertension | 401–405, 437.2 |
| DM | 250, 357.2, 362.0, 366.41 |
| Hyperlipidemia | 272.0–272.4 |
| CHF | 428 |
| Chronic kidney disease | 585 |
| Peripheral artery disease | 443, 444 |
| Prior stroke | 430–438 |
| Dementia | 290, 294.1, 294.8, 331.0, 331.1, 331.8 |
| Parkinson’s disease | 332 |
| Acute coronary syndrome | 410, 411, 413 |
| Adverse outcome | |
| Coronary artery bypass grafting | 36.1 |
| Cerebrovascular events | 430–436 |
| Ischemic stroke (artery occlusion) | 433, 434 |
| Hemorrhagic stroke | 431, 432 |
| Transient ischemic attack | 435 |
| Subarachnoid hemorrhage | 430 |
| Acute myocardial infarction | 410 |
Abbreviations: CHF, congestive heart failure; DM, diabetes mellitus.