Abstract
Background and Objective:
Non-scarring alopecia is a challenge in the diagnosis and treatment, rarely studied in Asian countries.
The current study aimed at evaluating histopathological features including hair count of different subtypes of non-scarring alopecia in Iranian patients.
Methods:
The current study was conducted on 114 cases diagnosed with non-scarring alopecia in Molecular Dermatology Research Center and Pathology Department of Shiraz University of Medical Sciences, Shiraz, Iran. Cases with two 4-mm scalp punch biopsies were selected. Patients’ clinical data were compared with histological findings.
Results:
Androgenetic alopecia (AGA) was the most common subtype followed by alopecia areata (AA) and combined AGA/telogen effluvium (TE). Perifollicular inflammation was observed in 21% of AGA with a significant difference in males and females (66.7% vs. 33.3%; P-value <0.05). Clinical and histopathologic diagnoses were correlated in 55% of cases. Maximum correlation was observed in combined AGA and chronic TE (88%). For vertical sections, the diagnostic rate was 33.6%, while 88% for transverse sections.
Conclusion:
Transverse together with vertical sectioning provides most of the information in non-scarring alopecias, while transverse sectioning is enough to diagnose the majority of non-scarring alopecias. Perifollicular inflammation was observed in a significant number of cases with AGA, more common in males. It is suggested to report such cases as possibly curable.
Key Words: Scalp, Biopsy, Pathology, Alopecia, Androgenetic alopecia, Alopecia areata, Iran
Introduction
Primary alopecia of the scalp is usually divided into scarring and non-scarring groups. Androgenetic alopecia (AGA), telogen effluvium (TE), alopecia areata (AA), trichotillomania, and traction alopecia are among the most common types of non-scarring hair loss. The histopathological interpretation of scalp biopsy specimens of the patients with alopecia may be complicated, even for dermatopathologists, since it requires proper clinical information, adequate tissue sampling, and appropriate laboratory processing.
Recent studies recommend two 4-mm punch biopsy specimens including subcutaneous tissue for vertical and transverse sectioning to help the interpretation. Vertical sections are suitable to evaluate alopecias with interface changes, lichenoid infiltrate, and subcutaneous pathologies; however, they only show about 10% of hair follicles present in the specimen. Transverse sectioning is useful to detect focal follicular pathology; moreover, it provides quantitative data of follicular cycling, as well as morphometric evaluation of the hair follicles throughout their entire length, from the bulb to the infundibulum (1-5). Most subtypes of non-scarring alopecia have similar clinical presentations albeit with different treatment protocols; therefore, an accurate histopathological diagnosis is needed for more effective treatment. The current study aimed at evaluating histopathological features of non-scarring alopecia cases for which definite diagnosis needs histopathologic examination. The incidence of each subtype was evaluated, and the histologic features of male and female AGA and AA were compared. The correlation of histologic findings of vertical sections with those of transverse ones was studied, and finally the correlation of histologic findings with clinical impression was evaluated.
Material and methods
The current cross sectional study was conducted from 2010 to 2015. All scalp biopsies diagnosed as non-scarring alopecia were selected from Major Dermatopathology Laboratory of Shiraz University of Medical Sciences, Shiraz, Iran. The cases suspected to non-scarring alopecia with two 4-mm punch biopsies were included in the study. The patients’ clinical information including age, gender, special physical examination findings, and clinical differential diagnosis were also collected. Cases with incomplete clinical information or only one punch biopsy and also the ones with inappropriate cut sections (tangential cut) were excluded from the study.
The protocol for scalp biopsy was two 4-mm punch biopsies at the most involved areas of the scalp; ie, often central mid scalp in females and frontal or vertex in males in symmetrical position. The biopsy specimens were fixed in 10% neutral buffered formalin (NBF) solution. One of the specimens was used for horizontal sectioning. This specimen was bisected 1-1.5 mm below the epidermal surface. The two cut surfaces were labeled by eosin, wrapped in filter paper, and embedded side by side in a capsule. The other specimen was bisected and sectioned parallel to the hair follicle. The blocks were processed routinely and sectioned serially by an expert technician. At least six vertical and 12 horizontal cut sections were prepared. Each level of infundibulum, isthmus, and bulbar area were carefully evaluated, separately. Diagnosis of different types of non-scarring alopecia was based on criteria used by Sperling et al. (6).
Quantitative data of transverse cut sections including total hair count, terminal and vellus hairs, the ratio of terminal to vellus (TVR), and anagen to telogen ratio percentage (ATR). Size difference of hair follicles, distorted hair follicles, inflammation (perivascular, parifollicular, and peribulbar), hemorrhage, and pigment cast were also collected. Epidermal change, peribulbar inflammation, sign of miniaturization, estimation of hair count, catagen/telogen (C/T), vellus hairs, and follicular streamers were investigated in vertical sections. To determine TVR, indeterminate hair was included in the terminal count as proposed by Whiting et al. (7). The cases with histologic features of decreased hair count, miniaturization of follicles with varying sized follicles, and increased catagen and telogen follicles of ≥ 30% were considered as combined AGA and chronic TE (combined AGA/TE).
Male and female non-scarring alopecia in common subtypes were compared. Vertical and transverse sections and also correlation with clinical differential diagnoses were statistically analyzed using t and chisquare tests.
Results
Totally, 114 scalp biopsies with non-scarring alopecia were studied. The gender distribution ratio, female [90(79%)] to male [24(21%)], was 3.75/1. Age and gender distribution in each subtype of non-scarring alopecia are summarized in Table 1.
Table 1.
Prevalence and Demographic Data of Different Subtypes of Non-scarring Alopecia
| Subtype | Total |
Female |
Male |
Age Range, yr |
||||
|---|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | Female | Male | |
| Androgenetic alopecia | 61 | 53.5 | 46 | 75.5 | 15 | 24.5 | 11-50 Mean:29 |
18-37 Mean:26 |
| Alopecia areata | 20 | 17.5 | 15 | 75 | 5 | 25 | 15-35 Mean:24 |
13-47 Mean:27 |
| Combined alopecia AGA/TE | 19 | 16.7 | 17 | 89.4 | 2 | 10.6 | 19-45 Mean:27 |
18-35 Mean:25 |
| Trichotillomania | 5 | 4.4 | 3 | 60 | 2 | 40 | 18-32 Mean:25 |
8-25 Mean:17 |
| Traction alopecia | 1 | 0.9 | 1 | 100 | - | - | 25 | - |
| sp Non-cific | 8 | 7 | 8 | 100 | - | - | 16-33 Mean:25 |
- |
AGA/TE, androgenetic alopecia to telogen effluvium
The hair count results in AGA were: total hair count= 23.5, TVR= 3.7/1 and ATR= 80/20. While the same figures for AA were 16.5, 5.9/1, and 60/40, respectively. In combined AGA/TE, the total count was 22.5, TVR: 4.1/1, and ATR: 63/37. These findings were compared between males and females within subtypes of AGA and AA. There was no significant difference between the total hair count, terminal, vellus, TVR, and ATR in the two subtypes (P >0.05). Epidermal change was observed in 10% of AGA cases as the most common subtype.
The changes were spongiotic reaction (73%), nonspecific hyperkeratosis and/or acanthosis (18%), and follicular plugging (9%). Histopathologic features of non-scarring alopecia in vertical cut sections and their prevalence are summarized in Table 2.
Table 2.
Histopathologic Features of Different Types of Non-scarring Alopecia in Vertical Sections
| Histological Features | Androgenetic Alopecia | Alopecia Alopecia Areata | Combined AGA/TE | Trichotillomania |
|---|---|---|---|---|
| Decreased total count | 78% | 85% | 57% | 100% |
| Increased C/T follicle | 54% | 75% | 57% | 100% |
| Increased vellus hair | 60% | 10% | 21% | --- |
| Miniaturization | 32% | --- | 26% | --- |
| Perifollicular inflammation | 21% | 4% | 5% | 18% |
| Perivascular inflammation | 48% | 6% | 10% | 12% |
| Pigment cast | --- | 25% | --- | 80% |
| Follicular streamer | 18% | 25% | 21% | --- |
| Peribulbar inflammation | --- | 50% | --- | --- |
| Hemorrhage | --- | --- | --- | 20% |
| Distorted hair follicle | --- | --- | --- | 20% |
AGA/TE, androgenetic alopecia to telogen effluvium; C/T, catagen/telogen
The results of the total count, TVR, ATR, and perifollicular inflammation in the two most common subtypes of non-scarring alopecia (AGA and AA) in males and females were compared. There was no significant difference between the hair count values and ratios in these subtypes (P >0.05). Perifollicu lar inflammation in AGA was observed in 35.7% of females and 66.7% of males (P <0.05), presented in Table 3. In AA, there was no significant difference in the presence of perifollicular inflammation between males and females.
Table 3.
Comparison of Hair Count and Perifollicular Inflammation in Males and Females with Androgenetic Alopecia
| Histologic Features | Female AGA | Male AGA | P-value |
|---|---|---|---|
| Total count | 21.7 | 25.3 | >0.05 |
| T:V ratio | 4/1 | 3.5/1 | > 0.05 |
| A:T ratio | 80/20 | 79/21 | >0.05 |
| Perifollicular inflammation | 35.7% | 66.7% | 0.02 |
AGA, androgenetic alopecia; T:V, transverse to vertical; A;T, anagen to telogen
The histological findings of vertical sections, without considering transverse sections, were divided into three descriptive categories as follows:
diagnostic (having a pathognomic feature)
-not diagnostic (having some diagnostic clues, but not definitive)
-non-specific
These categories were compared with final diagnosis (considering both vertical and transverse sections). Findings of the vertical sections alone were diagnostic in 36.8%, not diagnostic in 10.5%, and non-specific in 52.6% of patients with non-scarring alopecia. Within different subtypes, vertical sections were mostly diagnostic in trichotillomania (83%), followed by AA (57%), and AGA (29%). The diagnostic rate of transverse sections was higher in all subtypes compared with those of the vertical sections, 85% for trichotillomania, 88 % for AA and 90% for AGA, with overall diagnostic rate of 88%.
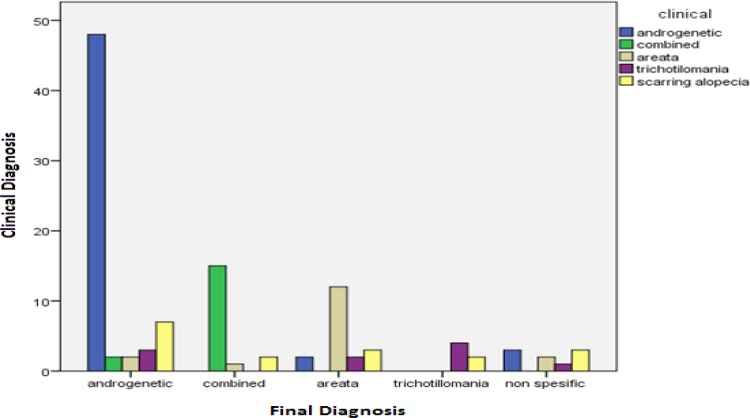
In 85% of the cases, the final diagnosis agreed with one of the first two clinical differential diagnoses. While in 55% of the cases, the final diagnosis was exactly the first clinical differential diagnosis. Maximum number of accurate clinical diagnoses was combined AGA/TE (83%) followed by AGA (77%), trichotillomania (66%), and AA (63%). Scarring alopecia was among the differential diagnosis in 15% of the cases.
Figure 1.
Comparison of Clinical Differential Diagnosis With Final Diagnosis Among Different Subtypes of Non-scarring Alopecia
Discussion
Non-scarring alopecia is a common dermatologic problem, which usually needs histopathologic confirmation and proper treatment. In the current study, AGA, AA, TE, and trichotillomania were the common subtypes. AGA was the most common type both in males and females followed by AA and combined AGA/TE. The mean age of the patients was higher in females with AGA, AA, and combined AGA/TE subtypes. Mean age of Iranian patients in the current study was lower than those of Europeans and Asians in AGA. Age distribution in AA was similar to that of the study in Singapore (1,9-11).
The current study findings were compared with those of Horenstein et al., in Table 4. The obtained results showed an overlap of hair count and ratio across subtypes. TVR was mostly affected in AGA, with a value of 1.6. They found a TVR of 2/1 in males (N = 14) and 1.5/1 in females (N = 81) (12). In another study on male and female AGA pattern, the TVR was 0.6–0.8 in 26 male cases and 1.7–1.9 in 94 female cases (13). A study on males showed TVR value of 1.7/1 (N = 106) in AGA pattern (14). While another study on females showed TVR value of 2.6/1 (N = 80) in AGA pattern (15). Authors` previous study showed TVR values in male and female AGA pattern as 3.1/1 (N = 25) and 2.9/1 (N = 28), respectively (8).
Table 4.
Follicular Count and Ratio in Non-scarring Alopecia
| Follicular Count and Ratio in Non-scarring Alopecia | ||||||
|---|---|---|---|---|---|---|
| Study |
The Current Study (Iranian) |
Horenstein et al. (American Whites) |
||||
| Histologic features | TC** | TVR | ATR | TC | TVR | ATR |
| Androgenetic alopecia | 23.5 ± 0.6 | 3.7 ± 0.1 | 4 ± 0.3 | 25.5 ± 1.0 | 1.6 ± 0.1 | 5.4 ± 0.5 |
| Alopecia areata | 16. 5± 1.0 | 5.9 ± 0.2 | 1.5 ± 0.2 | 22.3 ± 0.9 | 3.2 ± 0.4 | 1.6 ± 0.4 |
| Combined alopecia* | 22.5 ± 1.0 | 4.1 ± 0.4 | 1.7 ± 1.0 | - | - | - |
| Trichotillomania | 29.5 ± 0.5 | 6 ± 0.5 | 2.8 ± 0.4 | 27.1 ± 2.6 | 5.8 ± 1.4 | 2.8 ± 1.7 |
Combined alopecia
, androgenetic/telogen effluvium; TC
, total hair count; TVR, the ratio of terminal to vellus; ATR, anagen to telogen ratio percentage
In the AGA group, 32% of the cases had TVR of ≥4/1 and were diagnosed with AGA according to other histopathological criteria of this category, mainly miniaturization of hair follicles with clinical impression of AGA as the first one. Eight cases had TVR of ≥7/1, which were in favor of very early stage of androgenetic alopecia. This agreed with the results of the authors` previous study (8). In another study, the patients presenting with very early stages of alopecia had a TVR of 7/1 (n = 12) and the ones with more established disease had a TVR of 3/1 (n = 7), demonstrating the progressive decrease in TVR with advancing disease (16).
There were some cases of non-scarring alopecia with overlapping patterns of AGA and TE. It was difficult to differentiate such cases from pure AGA according to history, physical examination, and hair pull test results. Meanwhile, histologically, the combined features of miniaturization, and prominent increase in C/T follicles were in favor of combined AGA/TE. Telogen follicles increased in AGA up to 15%-20%, but values more than 30% were characteristics of TE (17,18,19). Therefore, C/T counts of ≥30% with simultaneous varying sized and miniaturized follicles were considered as combined AGA/TE in the current study.
Several studies highlighted the value of transverse sectioning to diagnose non-scarring alopecia, but the recent approach is to take two 4-mm scalp punch biopsies when clinically suspicious to non-scarring alo pecia (20,21,22). The current study evaluated histological features separately in the vertical sections, and then, both in vertical and transverse sections. Vertical sections were diagnostic only in 36% of cases; trichotillomania and AA had the highest rates (83% and 57%, respectively) and AGA the lowest rate (29%). It shows the necessity of simultaneous transverse sectioning, especially in the cases with clinical impression of AGA. The result can also guide the pathologists on how to handle the scalp specimen (vertical vs. transverse sectioning), when only one biopsy is submitted. Ozcan et al., also concluded that it may be preferable to have transverse sections in the cases of suspected non-cicatricial alopecia, and vertical sections in cases of suspected lichen planopilaris (23). In a recent systematic review and meta-analysis on diagnostic value of horizontal versus vertical sections for scarring and non-scarring alopecia, the pooled diagnostic rates were 0.81 (95% confidence interval (CI): 0.70-0.92) and 0.76 (95%CI: 0.60-0.93), respectively, with extensive heterogeneity among these studies. To diagnose scarring alopecia, there were three horizontal and five vertical sectioning studies. The pooled diagnostic rates were 0.86 (95%CI: 0.66-1) and 0.90 (95%CI: 0.82-0.98), respectively, and heterogeneity was also observed. They concluded that no significant difference existed between horizontal and vertical sectioning techniques to diagnose alopecia (24). Results of the current study showed increased follicular streamers in the cases of AA, combined AGA/TE, and AGA in concordance with other studies (25,26).
In some previous studies, the role of perifollicular inflammation in the pathogenesis and lower response rate to medical treatment and transplant surgery in AGA were discussed. Involution of the pilosebaceous unit, and sustained microscopic perifollicular inflammation with connective tissue remodeling, eventually resulted in permanent hair loss. It may be considered a possible cofactor in the complex etiology of AGA (22,27,28,29). The rate of perifollicular lymphohistiocytic inflammation in AGA, as observed in the literature, was 30% and 33% in the studies by Whiting (22), and Nirmal (30), respectively; while the rate of perifollicular inflammation in the current study was 21% in patients with AGA with a significant difference between males and females, presented in 66.7% of males and 35.7% of females. Comparison between perifollicular inflammation in males and females with AGA was rarely performed.
As reported in other studies, hair density was different according to the ethnic background, and there was a slight male and female difference in follicular counts, and it was recommended that the hair count and ratios be interpreted in comparison to normal population (31). In the current study, 32% of patients had TVR of 4/1 or more with other histologic features in favor of AGA (for example, miniaturization and marked size difference of hair follicles). This confirmed the fact that TVR of normal Iranians was higher than that of the textbook values (8).
No statistically significant difference was observed in hair count between males and females both in AGA and AA groups. The diagnosis and differentiating subtypes of non-scarring alopecia are a major challenge in dermatology practice when it is diffuse, especially in females. Usually, a combination of history, physical examination, laboratory and histopathologic findings are required for a definite diagnosis. The current study compared the clinical impression with histopathologic diagnosis, and revealed that the dermatologist’s diagnosis was accurate in 55% of cases according to the final histopathologic diagnosis. The most concordance was observed among AGA and combined AGA/TE. This result agreed with those of previous studies, which proposed that the diagnosis of AGA was usually made clinically, and biopsy may be used to confirm the diagnosis in cases with uncertain clinical diagnosis (4). In 15 % of cases, scarring alopecia was also mentioned in the differential diagnoses list, emphasizing the fact that the histopathologic confirmation was highly recommended in most cases of non-scarring alopecia except AGA.
Conclusion
Androgenetic alopecia was the most common type of non-scarring alopecia among patients referred to Shiraz University affiliated hospitals. Combined transverse and vertical sectioning provided most information in non-scarring alopecias, and transverse sectioning was recommended in situations in which Non-scarring Alopecia in Iranian Patients the pathologist received only one punch biopsy with clinical impression of non-scarring alopecia. Perifollicular inflammation was present in a significant number of AGA cases, more common in males, and it was suggested that it should be mentioned in pathology reports for possible therapeutic consideration. Correlation of clinical differential diagnoses with histopathologic findings was observed in half of the cases, indicating the necessity for histopathologic confirmation in most cases of non-scarring alopecia.
Acknowledgements:
The authors would like to thank the Center for Development of Clinical Research at Nemazee Hospital and Dr. Nasrin Shokrpour for editorial assistance.
Conflict of interest
The authors declare that there was no conflict of interest.
References
- 1.Su LH, Chen LS, Chen HH. Factors associated with female pattern hair loss and its prevalence in Taiwanese women: a community-based sur- vey. J Am Acad Dermatol. 2013;69(2):69–77. doi: 10.1016/j.jaad.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 2.Stefanato CM. Histopathology of alopecia: a clinicopathological approach to diagnosis. Histopathology. 2010;56(1):24–38. doi: 10.1111/j.1365-2559.2009.03439.x. [DOI] [PubMed] [Google Scholar]
- 3.Trüeb RM. Systematic approach to hair loss in women. JDDG: Journal der Deutschen Derma- tologischen Gesellschaft. 2010;8(4):284–97. doi: 10.1111/j.1610-0387.2010.07261.x. [DOI] [PubMed] [Google Scholar]
- 4.Werner B, Mulinari-Brenner F. Clinical and histological challenge in the differential diag- nosis of diffuse alopecia: female androgenetic alopecia, telogen effluvium and alopecia area- ta-Part II. Anais brasileiros de dermatologia. 2012;87(6):884–90. doi: 10.1590/S0365-05962012000600010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sellheyer K, Bergfeld WF. Histopatho- logic evaluation of alopecias. Am J Der- matopathol. 2006;28(3):236–59. doi: 10.1097/00000372-200606000-00051. [DOI] [PubMed] [Google Scholar]
- 6.Leonard C. Sperling, Shawn E. In: Cowper , Eleanor A, editors. An Atlas of Hair Pathology with Clinical Correlations. 2nd ed. Informa Health- care, 119 Farringdon Road, London EC1R 3DA, UK; 2012. [Google Scholar]
- 7.Whiting DA, Templeton SF, Solomon AR. Disorder of cutaneous appendages. In: Burnhill R, ed , editors. Text book of dermatopathology. New York: MC Grawhill; 2000. p. 201. [Google Scholar]
- 8.Aslani FS, Dastgheib L, Banihashemi BM. Hair counts in scalp biopsy of males and females with androgenetic alopecia compared with normal subjects. Journal of cutaneous pathology. 2009;36(7):734–9. doi: 10.1111/j.1600-0560.2008.01149.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee W-S, Lee H-J. Characteristics of androge- netic alopecia in Asian. Annals of dermatology. 2012;24(3):243–52. doi: 10.5021/ad.2012.24.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takashima I, Iju M, Sudo M. Alopecia androgeneticaits incidence in Japanese and associ- ated conditions. Hair Research: Springer; 1981. pp. 287–93. [Google Scholar]
- 11.Tan E, Tay YK, Goh CL, Chin Giam Y. The pat- tern and profile of alopecia areata in Singapore– a study of 219 Asians. International Journal of Dermatology. 2002;41(11):748–53. doi: 10.1046/j.1365-4362.2002.01357.x. [DOI] [PubMed] [Google Scholar]
- 12.Horenstein MG, Bacheler CJ. Follicular density and ratios in scarring and nonscarring alopecia. The American Journal of Dermatopathology. 2013;35(8):818–2613. doi: 10.1097/DAD.0b013e3182827fc7. [DOI] [PubMed] [Google Scholar]
- 13.Whiting DA, Waldstreicher J, Sanchez M, et al. Measuring reversal of hair miniaturization in androgenetic alopecia by follicular counts in horizontal sections of serial scalp biopsies: results of finasteride 1 mg treatment of men and postmenopausal women. J Invest Derma-tol Symp Proc. 1999;4:282–284. doi: 10.1038/sj.jidsp.5640230. [DOI] [PubMed] [Google Scholar]
- 14.Whiting DA. Diagnostic and predictive value of horizontal sections of scalp biopsy specimens in male pattern androgenetic alopecia. J Am Acad Dermatol. 1993;28:755–763. doi: 10.1016/0190-9622(93)70106-4. [DOI] [PubMed] [Google Scholar]
- 15.Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral ntiandro- gens. Br J Dermatol. 2005;152:466–473. doi: 10.1111/j.1365-2133.2005.06218.x. [DOI] [PubMed] [Google Scholar]
- 16.De Lacharriere O, Deloche C, Misciali C, et al. Hair diameter diversity: a clinical sign reflect- ing the follicle miniaturization. Arch Dermatol. 2001;137:641–646. [PubMed] [Google Scholar]
- 17.Robeora A, Guarrera M, Baldari M, Vecchio F. Distingushing androgenetic alopecia from telogen effluvium when associated in the same patient: a simple noninvasive method. Arch der- matol. 2005;141(10):1243–45. doi: 10.1001/archderm.141.10.1243. [DOI] [PubMed] [Google Scholar]
- 18.Whiting DA. Scalp biopsy as a diagnostic and prognostic tool in androgenetic alopecia. Dermathol Ther. 1998;8:24–33. [Google Scholar]
- 19.Shirvasta SB. Diffuse hair loss in adult female:Approach to diagnosis and management. Indian J dermatol venerol leporal. 2009;75(1):20–8. doi: 10.4103/0378-6323.45215. [DOI] [PubMed] [Google Scholar]
- 20.Jameel K, Ejaz A, Sohail M, Rahman SB. Val- ue of transverse section scalp biopsy in alope- cia areata-a clinicopathological correlation. J Coll Physicians Surg Pak. 2008;18(6):338–41. [PubMed] [Google Scholar]
- 21.Elston DM, Ferringer T, Dalton S, Fillman E, Tyler W. A comparison of vertical versus transverse sections in the evaluation of alope- cia biopsy specimens. Journal of the American Academy of Dermatology. 2005;53(2):267–72. doi: 10.1016/j.jaad.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Whiting DA. Diagnostic and predictive value of horizontal sections of scalp biopsy speci- mens in male pattern androgenetic alopecia. Journal of the American Academy of Deratology. 1993;28(5):755–63. doi: 10.1016/0190-9622(93)70106-4. [DOI] [PubMed] [Google Scholar]
- 23.Özcan D, Özen Ö, Seçkin D. Vertical vs transverse sections of scalp biopsy speci- mens: a pilot study on the comparison of the diagnostic value of two techniques in alope- cia. Clinical and experimental dermatology. 2011;36(8):855–63. doi: 10.1111/j.1365-2230.2011.04154.x. [DOI] [PubMed] [Google Scholar]
- 24.Xufeng DU1, Zhongming LI, Wenrong XU1, Xiaohui ZHOU2. Shaowen TANG3 Chuang SONG4 Weixin FAN4 Diagnostic value of horizontal versus vertical sections for scarring and nonscarring alopecia: a systematic review and meta-analysis. Eur J Dermatol. 2016;26(4):361–9. doi: 10.1684/ejd.2016.2797. [DOI] [PubMed] [Google Scholar]
- 25.Sinclair R, Jolley D, Mallari R, Magee J, Tosti A, Piracinni BM, et al. Morphological approach to hair disorders. Journal of Investi- gative Dermatology Symposium Proceedings. Nature Publishing Group; 2003. [DOI] [PubMed] [Google Scholar]
- 26.Horenstein MG, Jacob JS. Follicular stream- ers (stelae) in scarring and non‐scarring alopecia. Journal of cutaneous pathology. 2008;35(12):1115–20. doi: 10.1111/j.1600-0560.2008.00987.x. [DOI] [PubMed] [Google Scholar]
- 27.Mahé YF, Michelet JF, Billoni N, Jarrousse F, Buan B, Commo S, et al. Androgenetic alopecia and microinflammation. International journal of dermatology. 2000;39(8):576–84. doi: 10.1046/j.1365-4362.2000.00612.x. [DOI] [PubMed] [Google Scholar]
- 28.Magro CM, Rossi A, Poe J, Manhas-Bhutani S, Sadick N. The role of inflammation and im- munity in the pathogenesis of androgenetic alo- pecia. Journal of drugs in dermatology : JDD. 2011;10(12):1404–11. [PubMed] [Google Scholar]
- 29.Piérard G, Pierard-Franchimont C, Nik- kels-Tassoudji N, Nikkels A, Léger DS. Im- provement in the inflammatory aspect of an- drogenetic alopecia A pilot study with an antimicrobial lotion. Journal of dermatological treatment. 1996;7(3):153–7. [Google Scholar]
- 30.Nirmal B, Somiah S, Sacchidanand SA, Bi- ligi DS, Palo S. Evaluation of perifollicular inflammation of donor area during hair Transplantation in Androgenetic alopecia and its comparison with controls. Interna- tional journal of trichology. 2013;5(2):73. doi: 10.4103/0974-7753.122963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee H-J, Ha S-J, Lee J-H, Kim J-W, Kim H-O, Whiting DA. Hair counts from scalp biopsy specimens in Asians. Journal of the American Academy of Dermatology. 2002;46(2):218–21. doi: 10.1067/mjd.2002.119558. [DOI] [PubMed] [Google Scholar]